Combinatorial NeuroImaging Core Facility
The Combinatorial NeuroImaging (CNI) Core Facility at the Leibniz Institute for Neurobiology combines an outstanding range of imaging techniques for non-invasive human imaging, translational animal imaging and high resolution light microscopy.
As a modern research infrastructure and dialogue platform we aim at supporting our local and external users in their scientific plans and projects with high efficiency and expertise.
The scientific focus is directed at the combination of the different techniques from molecular to the systems level to gain a comprehensive knowledge of learning and memory processes. We refer to this integrative approach as "Combinatorial NeuroImaging".
Research Infrastructure
- Microscopy
The Combinatorial NeuroImaging core facility provides open access to state-of-the-art microscopes that support a large spectrum of imaging methods. We offer high-resolution multi-channel STED microscopy, lightsheet microscopy, FLIM microscopy, confocal microscopy and widefield microscopy. With the support of EFRE funding we built a new imaging unit for 2-photon imaging in behaving rodents. Furthermore, we support image analysis using professional software packages running on server workstations and a central processing server accessible via remote connections.
We are closely collaborating with GerBI-GMB, Society for Microscopy and Image Analysis and the Multi-parametric bioimaging and cytometry (MPBIC) core facility at the University of Magdeburg.
High-resolution MicroscopyInverse Leica TCS STED-SP8 3X
Stimulated emission depletion microscopy

- high resolution multi-channel STED in 3D
- multi-channel confocal microscopy
- live cell imaging
- tile-scan imaging
Confocal MicroscopyUpright Leica TCS SP8

- multi-channel confocal microscopy
- tile-scan imaging
Widefield Fluorescence MicroscopyLeica Thunder Imager

- multi-channel widefield microscopy
- brightfield and fluorescence microscopy
- allows computational clearing
Multi-Photon Microscopydiverse 2-Photon microscopes
Behavioral Bioimaging Core Unit
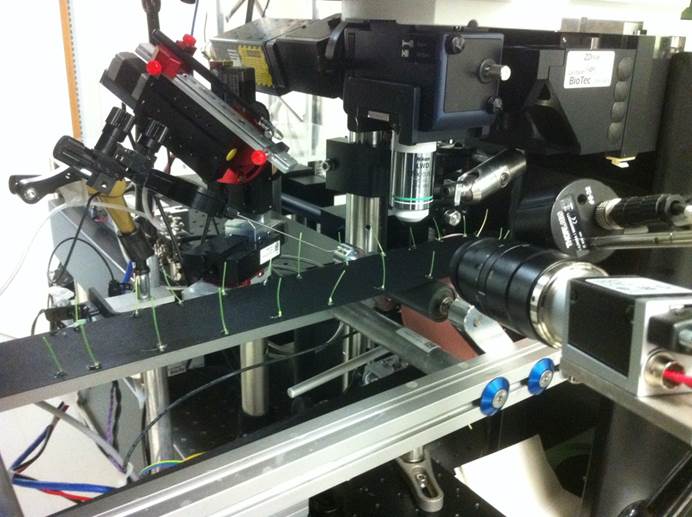
- in-vivo two-photon functional imaging of neuronal activity
- imaging awake headfixed mice on linear treadmill
- additional recording of behavioral and physiological metadata
Lightsheet MicroscopyMiltenyi Blaze

- 3D multi-channel imaging of diverse specimen (larvae, organs, adult mice)
- live imaging or fixed and cleared specimen (refractive indix range: 1.33 to 1.56)
- LightSpeed Scan Mode and MACS IQ 3D processing software (deconvolution, destriping etc.)
Leica SP8-Digital LightSheet Microscope
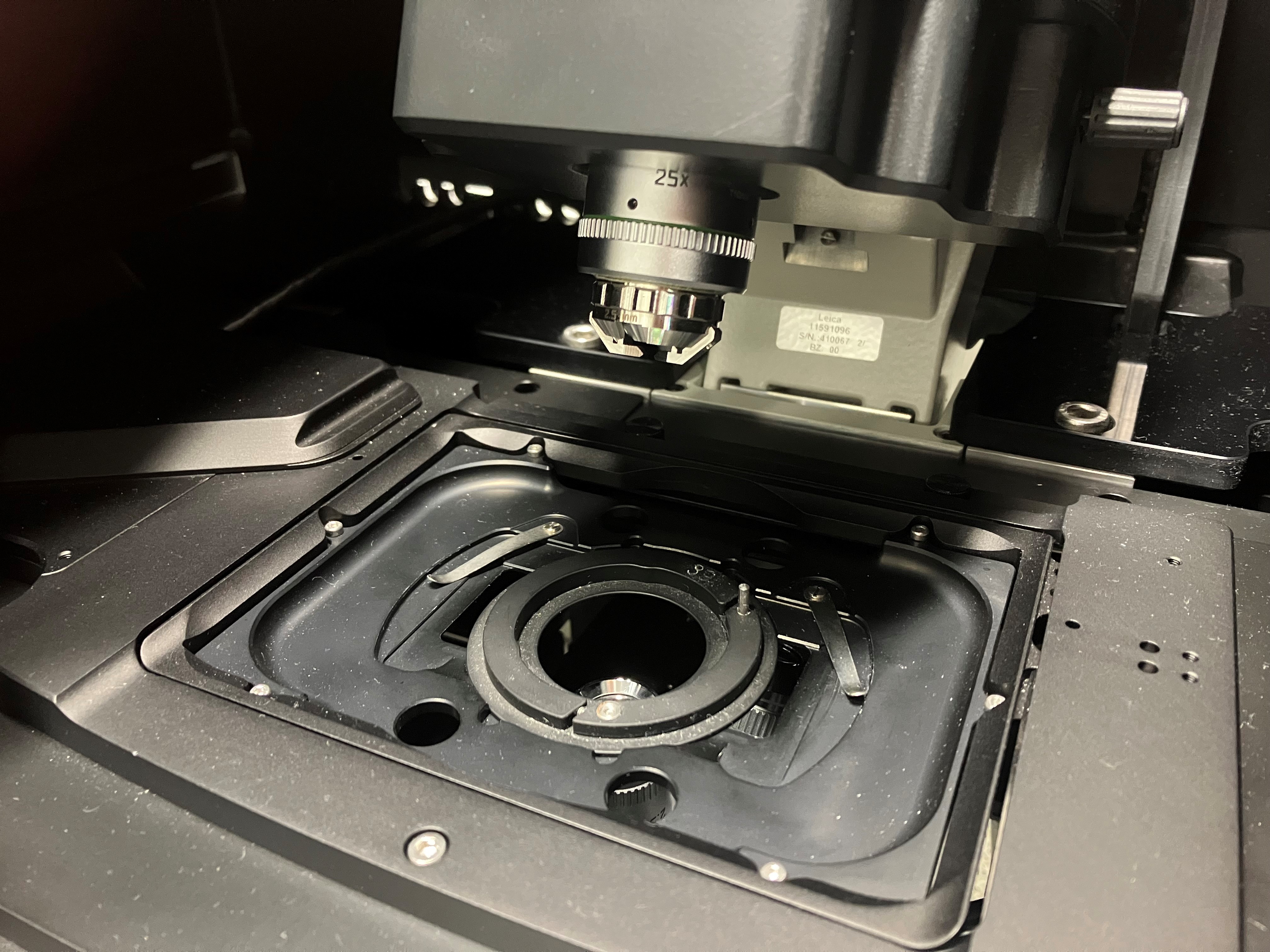
- fast sub-cellular imaging, live and fixed
- multi-channel 3D-FLIM imaging
Analysis SoftwareFor image processing and analysis the following software packages can be used at the LIN:
- Huygens Professional
commercial deconvolution and image analysis software - Imaris
commercial three-dimensional image analysis software - Arivis Vision 4D + InViewR
commercial three-dimensional image analysis software and virtual reality - ImageJ / Fiji
open-source image analysis software - MACS IQ 3D
commercial image processing software
These software solutions are available for multiple remote users on a Hive Processing Server.
- Small Animal Imaging
Animal imaging provides an important link between microscopy and human imaging. The available imaging techniques (MRI, SPECT/CT, PET) allow animal experiments that are comparable to studies performed on humans. In addition, animal experiments permit the combination with invasive methods in-vivo. Thus, "molecular imaging" allows to draw conclusions from physiological processes on the molecular level. The combination with pharmacological and electrophysiological methods is particularly revealing with respect to mechanistic explanations of neuronal processes.
The Combinatorial NeuroImaging Core Facility provides users access to small animal imaging labs equipped with a 9.4 Tesla MR scanner, a SPECT/CT system and a PET scanner. The labs offer the option of sequentially combining the different modalities in the same animal.
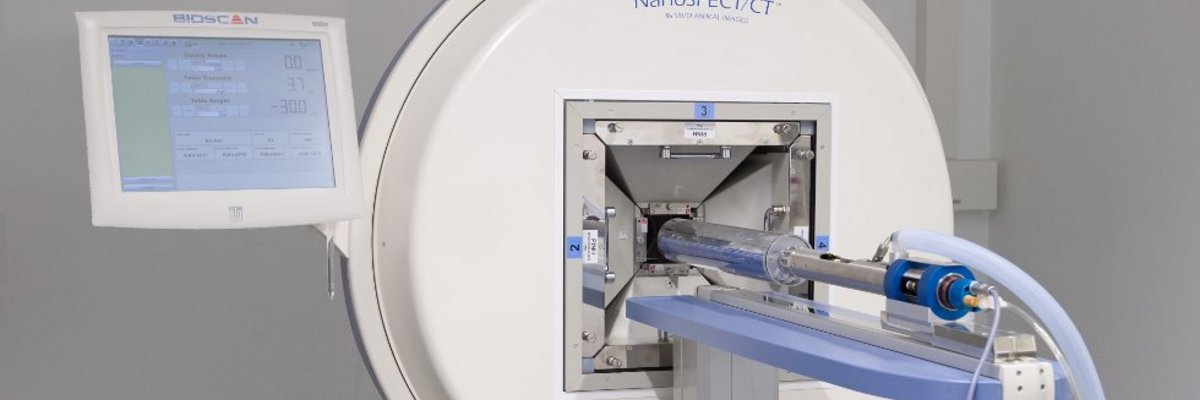
PET and SPECT/CTThe small-animal in vivo radionuclide imaging lab is equipped with a Philips Mosaic PET scanner and a NanoSPECT/CT scanner (Bioscan/Mediso). PET and SPECT make it possible to visualize the biodistribution of radioactively labeled compounds. The isotropic spatial resolution with the SPECT system at the CNI exceeds 500 µm. Classical fields of application are imaging of brain metabolism and function, neurotransmitter systems and receptor distributions as well as neurodegeneration and neuroinflammation.

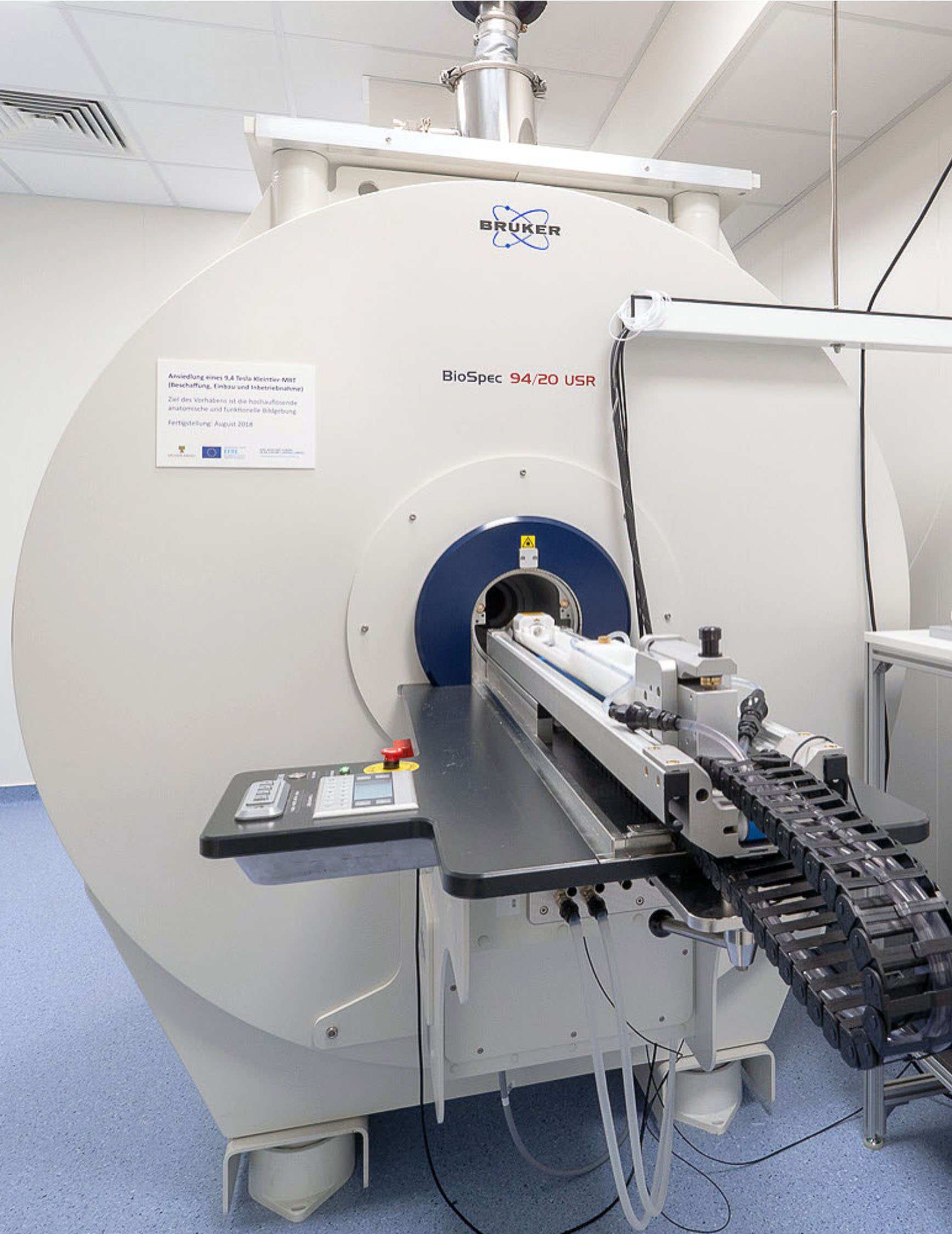
9.4 Tesla Small-Animal Magnetic Resonance ScannerIn 2018, the LIN has established an ultra-high field magnetic resonance scanner (Bruker BioSpec 94/20 UHF). This scanner is equipped with a cryocoil for high-resolution magnetic resonance imaging (MRI), providing an excellent signal-to-noise ratio, and with various RF-coils suited for combining MRI techniques (anatomy, functional MRI, spectroscopy, diffusion- and perfusion weighted imaging) with optogenetic, electrophysiological, pharmacological, and similar techniques in rodents.
- Human Brain Imaging
For ethical reasons, neuro-scientific research on humans is for the most part confined to non-invasive methods. However, constant innovation in imaging technology and image analysis allows increasingly detailed insight into the human brain.
Improving both spatial and temporal resolution is the driving force behind such technological developments. Additionally, combining different modalities, for example MRI with EEG or MRI with PET, will allow a much more comprehensive understanding of neuronal processes in the brain.
The Combinatorial NeuroImaging core facility offers open access to ultra high-field magnetic resonance tomography. We provide modern MR coil technology and all common MR sequences. For functional imaging, we provide high quality visual and auditory stimulation systems as well as monitoring devices for recording physiological parameters and the behavior of subjects.
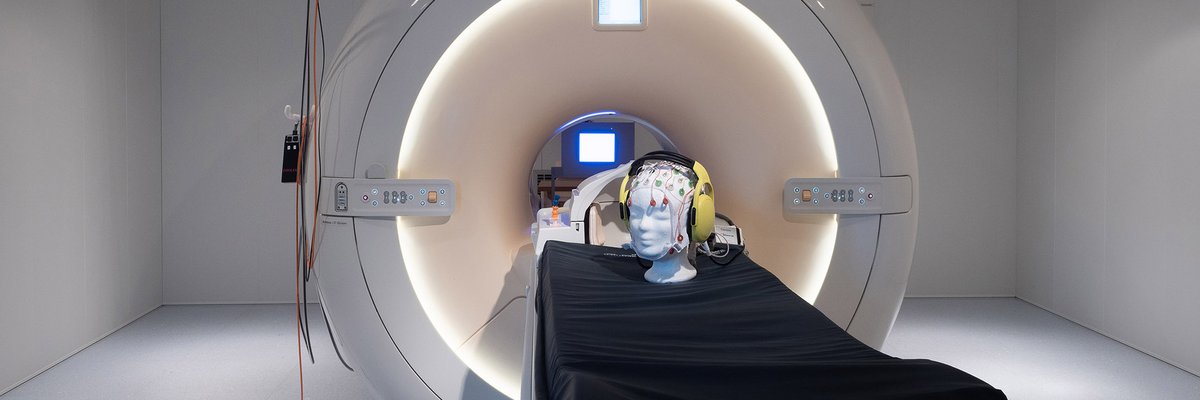
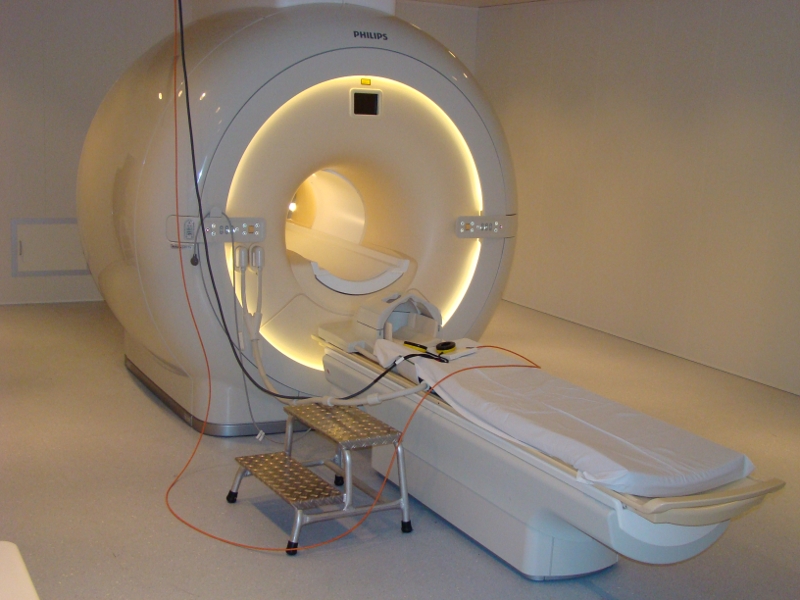
3 Tesla Magnetic Resonance ImagingFunded by the federal state of Saxony-Anhalt and the European Regional Development Fund (ERDF), the Philips 3T Achieva dStream was put into operation in November 2013. In addition to common methods for multiparametric anatomical imaging, the scanner is particularly suited for whole-brain fMRI (2 mm isotropic resolution) and simultaneous highly synchronous and artifact-reduced acquisition of EEG signals (64-channel system with carbon wire loop technology). The laboratory is characterized by numerous additional systems for the acquisition of respiration, heart rate, skin conductance, facial expression, pupil size, eye tracking and button-press dynamics. For detailed information about technical equipment at the scanner see our MRI-WIKI. When using the facility mind our lab rules.
ElectroencephalographyElectroencephalography (EEG) allows detecting the activity of neurons by the generated currents of electrically charged particles (like sodium and potassium) with a high temporal resolution of about one millisecond. An arrangement of special electrodes detects the spatial distribution of the electrical potential generated by the neuronal activity on the surface of the subject’s head. In our lab, several EEG are systems available, including an active 128-electrode device and a MR compatible system.
Moreover, EEG measurements may be combined with multiple peripheral physiological recording and stimulation methods. Multichannel electromyography, i.e. the measurement of the electrical activity of muscles, measurement of skin conductance and breathing movements, and electrocardiography may be used to capture information on emotional states. Eye tracking and the recording of otoacoustic emissions are possible as well. Transcranial electric stimulation can be used to directly modulate brain activity.

Sound proof chamberCNI operates a sound proof chamber for psychoacoustic studies of individual subjects. This includes audiometric measurement of hearing threshold using Madsen Itera II by Otometrics, characterization of basic auditory skills using MediTECH’s Brain-Boy, and speech comprehension using HörTech’s “Oldenburg Measurement Applications”.



Mock ScannerCNI provides access to a mock MRI scanner including equipment for the presentation of realistic scanner noise and auditory and visual stimuli. The scanner can be used for subjects to become more comfortable in the MRI environment, to train tasks for subsequent fMRI studies and adjust the difficulty level, which may be affected by lying on the back in a narrow and noisy MRI scanner.
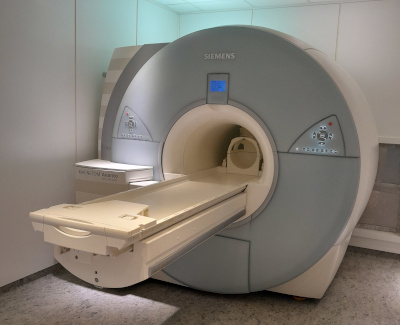
7 Tesla Magnetic Resonance ImagingThe Leibniz Institute for Neurobiology installed Europe's first 7 Tesla magnetic resonance scanner (MRI) for human studies in a dedicated building adjacent to LIN’s main building and operated it until 2021. In 2022, the device was transferred to the OVGU, but will continue to be available for use as usual.
The stronger magnetic field generates a greatly improved single to noise ratio which is about twice as good as that of a 3 Tesla MRI. This stronger signal can be traded for higher spatial resolution of an equal factor. Thus, finer structures of the brain can be studied both anatomically and functionally. Furthermore the higher signal can also be used to reduce the required scan time which is particularly useful for learning studies.

- Study Application
Study Application
Application Process
If you want to conduct a study on CNI devices please fill in the study application below. The form will then be available as a PDF file for download and print. Please submit the signed application via mail (CNI, Brenneckestr. 6, 39118 Magdeburg), fax (0391/626392589) or personally.
After an internal evaluation of your application according to the criteria given in the user rules, you will be informed about the acceptance of your study, your contact person(s) and the possible starting date for your project.
User Rules
The User Rules are the basis for all services offered by the CNI and have to be accepted by our users.
Changes of running studies
A new study application for a running study is necessary, if
the measuring time is exceeded or falls short by more than 10 %,
the funding changes, particularly in case you need more measuring time,
you want to address a new question/topic,
persons in charge of the project change.
Research Topics
- Metabolic Activity at Cellular and Systems Level
Neural as well as glial activity and metabolism are closely related. With increasing activity, the ion movements across mitochondria and cell membranes, as well as glucose and oxygen consumption, increase. Changes in neuronal and glial metabolism trigger changes in local blood flow.
Within the CNI we use an array of different techniques for imaging neuronal and cerebral metabolism on a macroscopic, mesoscopic and cellular level in order to uncover patterns ofneuronal activity associated with learning and memory-related processes as well as neurodegenerative diseases.
To study the relationship between neuronal activity and metabolic state, we observe:
changes in blood flow, glucose consumption and oxygen metabolism using SPECT, PET and fMRI
changes in potassium turnover by means of SPECT imaging and histochemical detection of the K+ probe thallium (Tl+)
the intensity and fluorescence lifetime of intrinsic coenzymes (e.g. NADH and FAD) in living neuronal cell cultures
Ca2+ imaging and pH changes
Metabolic Imaging at Cellular Level (Weber, Zuschratter)
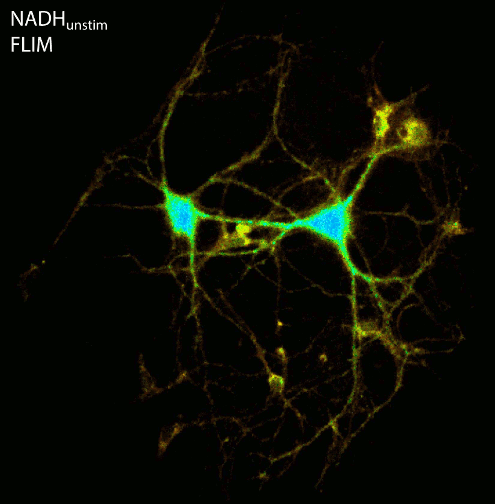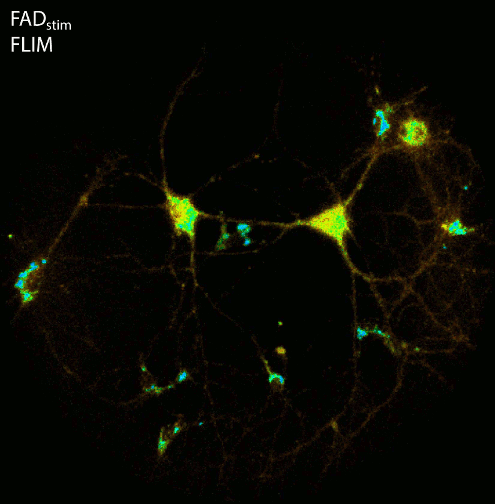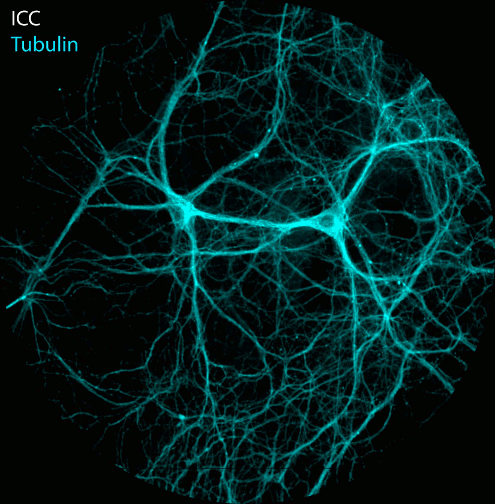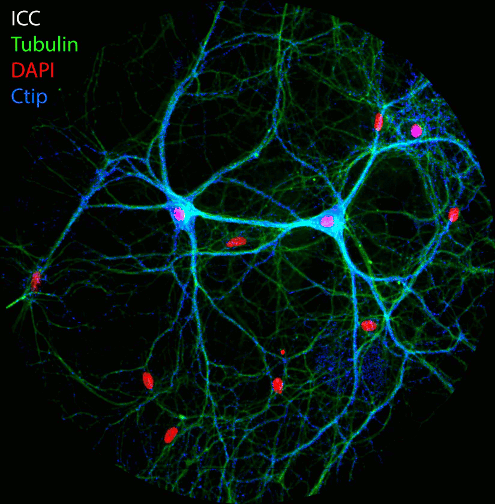
Cellular energy production in form of ATP depends on glycolysis and the electron transport chain within mitochondria. As a first step of this complex chain, NADH, which is intrinsically fluorescent (ex. 355nm, em. 460nm), is oxidized to the non-fluorescent NAD+. Measuring the fluorescence lifetime of NADH provides information about the microenvironment of the co-enzyme. Different cellular conditions change the ratio between bound and unbound NADH. Since free NADH has a much shorter fluorescence lifetime compared to protein bound NADH, FLIM can be used to discriminate different metabolic states of a cell, like starvation, oxygen deprivation or higher activity (e.g. cell stress, inflammation).
By using the ultra-sensitive LINCam in single photon counting mode we study the complex relation between energy metabolism and electrical activity of neuronal cell cultures. Practically, we monitor the autofluorescence of NAD(P)H and FAD up to hours under low light conditions in combination with electrical stimulation or recording of the electrical activity via multi-electrode arrays. Following burst-activity the energy consumption of neurons shows a temporary reduction of NADH fluorescence intensity as well as an increase in the fluorescence lifetime indicating that mainly free NADH of the cytoplasm is oxidized to the non-fluorescent NAD+ whereas protein/enzyme-bound NADH persists.
Because NADH acts in almost all cells as an intrinsic, non-invasive fluorescence probe that allows for the monitoring of the metabolic dynamics of living cells, there is a huge interest to use the redox couple NAD+/NADH and the fluorescence lifetime characteristics of NADH not only to study the energy turnover in respect to neuronal activity during synaptic plasticity (i.e. learning) but also for medical diagnostics (e.g. neuro-inflammation). Therefore, in future we will develop sensitive tools for the analysis of the relation between energy supply and cellular behaviour.
Figure: Imaging of NAD(P)H and FAD fluorescence of neuronal cell cultures reveals changes in fluorescence intensity and fluorescence lifetime after electrical stimulation. After live cell imaging, cell cultures were fixed and immunostained with antibodies against Tubulin, Dapi and the transcription factor Ctip2. FLIM and confocal image acquisition by Ezgi Altun.
Funding:- DFG SFB 854 TPZ
- BMBF T-CAM4Life FKZ: 13N12675
Collaborators:- LIN: Altun, E., Herrera-Molina, R., Kobler, O., Prokazov, Y., Thomas, U., Turbin, E.
- OvGU: Arens, Ch., Davaris, N., Hartig, R., Hauser, M., Müller, A., Sabel, B., Vollmer, M., Walles, H.
- MPI Magdeburg: Ivanov, I.
- Univ. Mainz: Bikbaev, A., Heine, M.
FLIM and FRET Measurements (Weber, Herrera-Molina, Zuschratter)
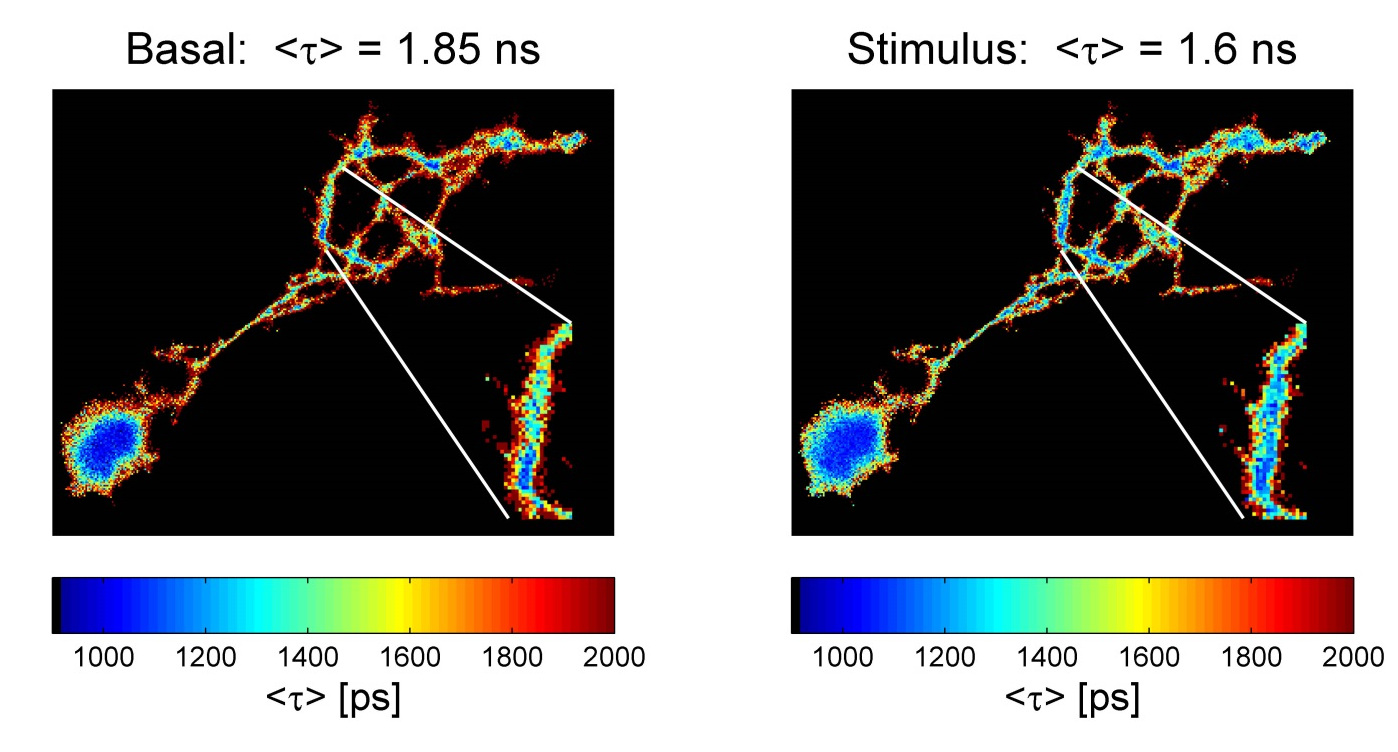
CNI performs FLIM based FRET measurements of biosensors to evaluate protein-protein interactions by single photon counting with LINCam. In addition, we image biosensors able to report on the concentration of biological relevant ions, i.e. calcium (Ca2+) and proton (H+) as indicators of changes in membrane potential and the intracellular pH due to neuronal activity.
Figure: Lifetime-based evaluation of pH changes in cultured neurons with the pH sensor (eGFP-pHsens). Left picture: basal condition; right, response to a robust stimulation (KCN, 200µM). A digital magnification highlights a dendritic segment where lifetime changes due to decreased pH occurred.
Collaborators:- National: Friederich, T. (TU Berlin)
- Magdeburg: Hartig, R., Müller, A., Schraven, B., Simioni, L. (Med Fac. OvGU)
- LIN: Gundelfinger, ED., Thomas, U.
Funding:- DFG CRC 854 TPZ
- BMBF TCAM4Life (FKZ: 13N12675)
In-vivo two-photon functional imaging of hippocampal neuronal activity (Fuhrmann, Bauer, Remy)
The hippocampus is involved in the encoding of episodic memories and hippocampal neuronal activity correlates to spatial information, self-motion, motivation and salience and it is tuned by prior experience or altered in disease conditions. We use in vivo two-photon microscopy combined with genetically-encoded indicators (e.g. genetically encoded calcium indicators GCaMP) to study the hippocampal neuronal circuit in awake behaving mice. This method allows to measure precise spatio-temporal activity patterns, target genetically labeled subpopulations and monitor sub-cellular compartments chronically in head-fixed mice performing spatial behaviors on a linear treadmill.
Video: Hippocampal GCamP6 expression with additional labeling of PV interneurons (red).Video: Recording of hippocampal activity (GCamP6) during locomotion.Imaging cerebral blood flow and potassium metabolism (Goldschmidt)
During the past decades a large variety of methods for imaging neuronal activity has been developed but it has remained difficult to assess brain-wide activation patterns in unrestrained behaving rodents. We have developed novel approaches and protocols for addressing this problem. Our methods are based on intravenously injecting rodents during ongoing behavior with tracers for imaging cerebral blood flow and K+-metabolism. The distribution of these tracers can be read out after injection either in vivo using single-photon emission computed tomography (SPECT) or in brain sections using histochemical methods. We have patented one of the tracers we use, the lipophilic chelate complex thallium diethyldithiocarbamate (TlDDC), for imaging cerebral K+-metabolism. Our novel approaches can provide images of brain-wide spatial activation patterns - averaged over time periods of a couple of minutes - in unrestrained behaving animals. The techniques are of high value not only for studying learning and memory. They also proof to be very useful for imaging pathological alterations in mouse models of neurological or psychiatric diseases.
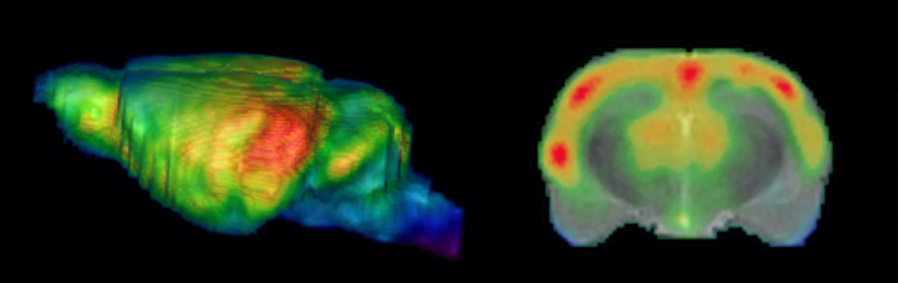
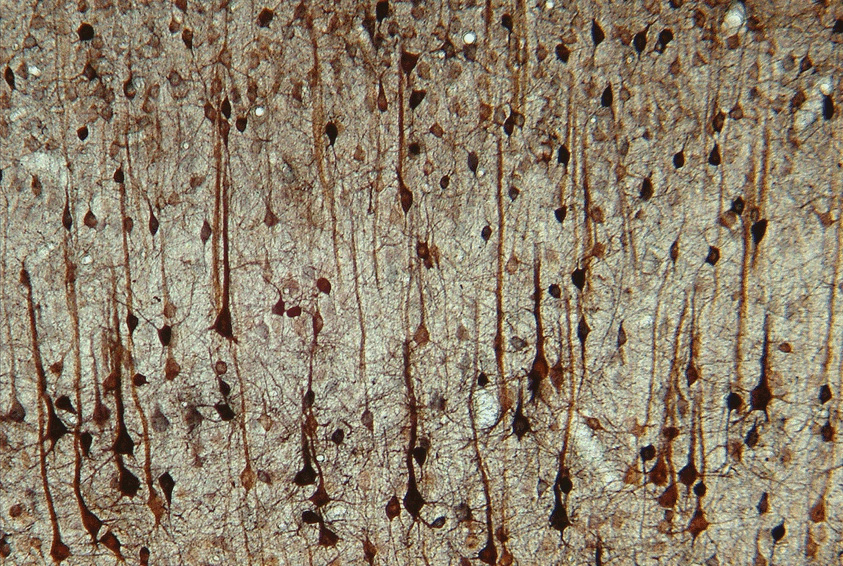 Figure: SPECT images of blood flow in rat brain during an auditory learning task. A 3D volume rendered whole-brain image of cerebral blood flow is shown on the left side, a frontal section at the level of the auditory cortex overlaid on an MR image on the right side (MR–image in grayscale, SPECT pseudocolored).Figure: Thallium uptake in layers IV and V rat somatosensory cortex.
Figure: SPECT images of blood flow in rat brain during an auditory learning task. A 3D volume rendered whole-brain image of cerebral blood flow is shown on the left side, a frontal section at the level of the auditory cortex overlaid on an MR image on the right side (MR–image in grayscale, SPECT pseudocolored).Figure: Thallium uptake in layers IV and V rat somatosensory cortex. - Functional Neuroanatomy
Translational Neuromodulation (Deliano)
The goal of our research activities is to modulate the dynamics of the brain via brain-machine interfaces (BMIs), in order to suppress pathological states. Moreover, we want to induce plastic network changes that sustainably eliminate pathological states without the need of permanent neuromodulation. In close translational collaboration with Lars Büntjen (OVGU University Hospital) we study common principles of clinical neuromodulation. We apply non-invasive, high-density EEG/MEG analysis in patients with well-defined pathological states treated by deep-brain or spinal cord stimulation (SCS). We draw on our longstanding expertise in thalamocortical neurodynamics and its electrical and pharmacological modulation both, in rodents and humans. Being hubs in the thalamocortical circuitry, we consider layer V pyramidal cells as common targets of various forms of clinical neuromodulation. They are major targets of dopaminergic modulation, and represent low-threshold sweet-spots of electric stimulation. We hypothesize that they are also involved in the generation of pathological thalamocortical oscillations observable in the EEG/MEG in various neuropsychiatric diseases. First results from patients receiving SCS-treatment of neuropathic pain, confirm that thalamocortical EEG-oscillations can be specifically linked to effects and side-effects of neuromodulation by SCS.
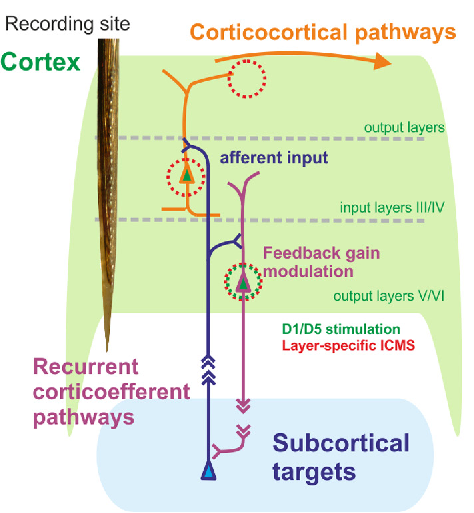 Figure: Layer-sepcific cortiocthalamic interface
Figure: Layer-sepcific cortiocthalamic interfaceCollaborators:
- Max Happel, AG CortXplorer, Department Systems Physiology of Learning
- Lars Büntjen, Department for Stereotactic Neurosurgery, OVGU University Hospital
Hemispheric interactions during auditory learning (Angenstein, Budinger, Michalek, Wenk)
The left and right auditory cortex are differentially specialized for the processing of distinct spectral and temporal acoustic features (e.g., frequency, duration), which lead, for example, to a left-hemispheric dominance for the processing of speech and species-specific vocalizations. Longstanding research at the LIN has demonstrated that common mechanisms exist in humans and rodents that underlie lateralized auditory cortex functions; however, the role of functional hemispheric interactions during auditory processing and in particular learning still remains unclear. Moreover, it is not understood how these interactions change due to a decline of the underlying anatomical prerequisites (corpus callosum, anterior commissure) and how dysfunctions of the interhemispheric connectivities may be compensated. Using an animal model of auditory learning (Mongolian gerbil), Go/No-Go behavioral paradigms, selective photolytic apoptosis of commissural neurons, and functional magnetic resonance tomography (fMRI) under binaural stimulation (including contralateral noise procedure) this project aims at revealing the effect of disruption of interhemispheric crosstalk during auditory learning. Thereby, the identification of compensatory mechanisms may lead to new therapeutic approaches for rehabilitation of patients with compromised hemispheric interaction (e.g., in the elderly) and/or with unilateral brain lesions (e.g., tumors).
Funding:
- LIN Special Project 2018 "Crosstalk between hemispheres during auditory learning: Disturbance and compensation"
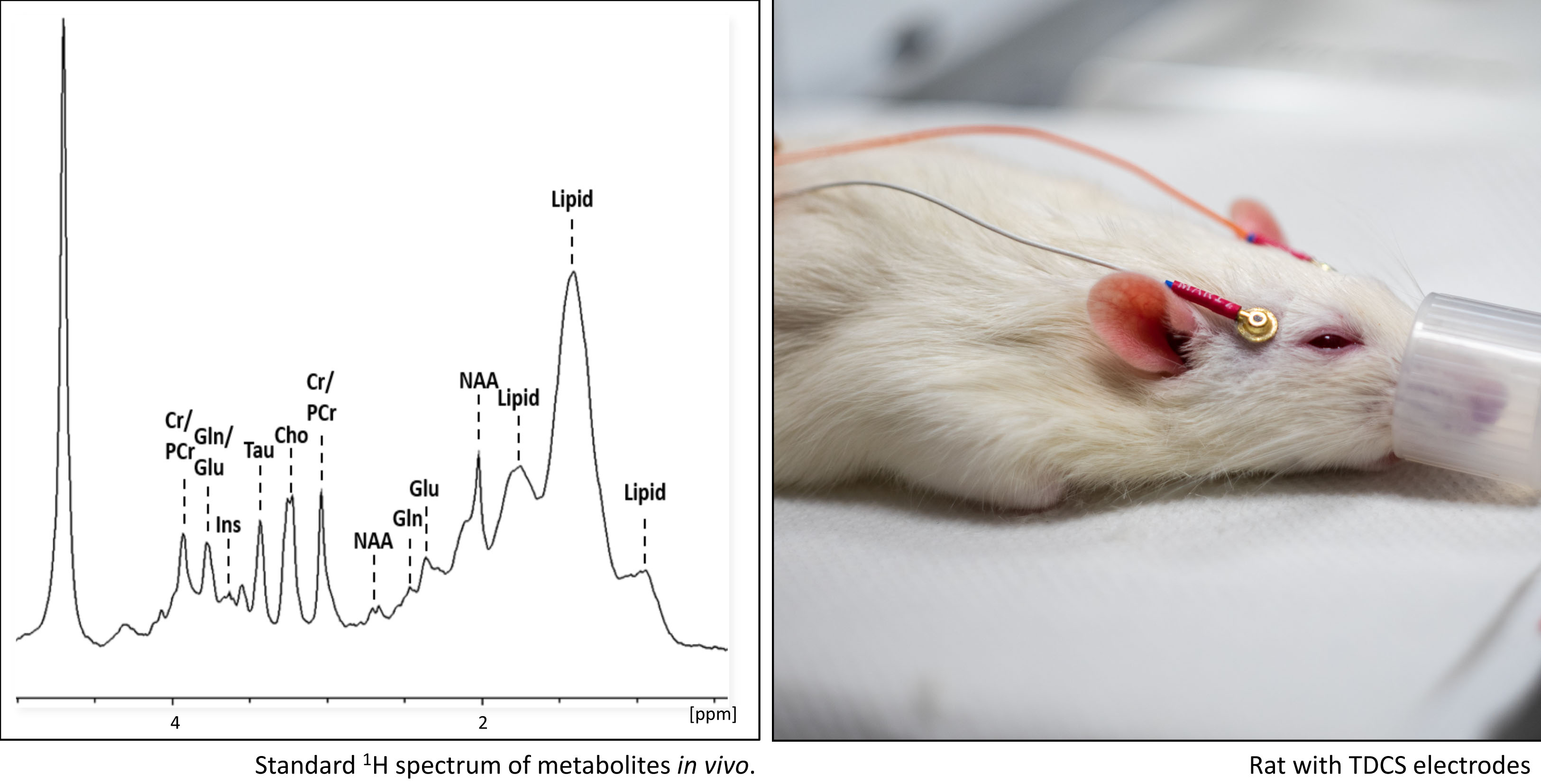
Functional anatomy of the brain during health and disease (Akter, Bhattacharjee, Budinger, Goldschmidt, Wenk)
Neuroinflammatory infectious diseases like cerebral malaria and toxoplasmosis as well as age-related neurodegenerations such as Parkinson and Alzheimer disease often lead to severe alterations of the brain structure and function. In several projects combining state-of-the-art immunohistological brain staining techniques and non-invasive small animal imaging like SPECT (single-photon emission computed tomography), MRI (magnetic resonance imaging) and MRS (magnetic resonance spectroscopy) we investigate the progress of these diseases and causal links between anatomical changes and specific dysfunctions of the brain. We also test diverse therapeutic approaches like pharmacological interventions and non-invasive TDCS (transcranial direct current stimulation).
Collaborators:
- Lisa Carius, Institute for Automation Engineering, Otto-von-Guericke University Magdeburg
- Philipp Ruhnau, Clinic for Neurology, Otto-von-Guericke University Magdeburg
- Dirk Schlüter, Nishanth Gopola, Institute for Medical Microbiology and Hospital Epidemiology, Hannover Medical School
Funding:- ABINEP - Analysis, Imaging, and Modelling of Neuronal and Inflammatory Processes; Project 2 (Modul 1): Development of new techniques for visualization of neuroinflammatory processes during infections and autoimmunity diseases of the brain.
- CBBS NeuroNetwork "Non-invasive Deep Brain Stimulation for Motor Disorders (NeeMo)"
Superresolution Microscopy (Kobler, Zuschratter)
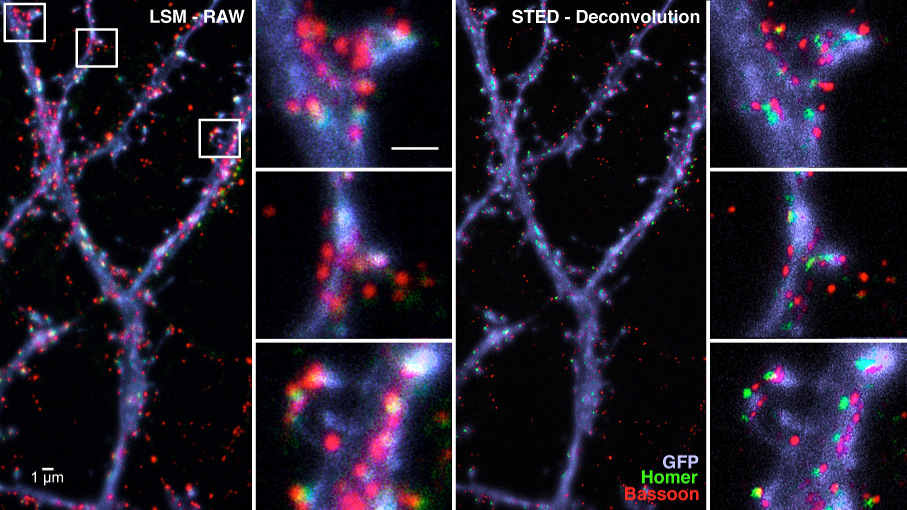
Microscopy beyond the diffraction limit has become very popular in recent years and many efforts have been done to improve the resolution limit down to few 10th of nanometer by taking advantage of the fact that fluorophores can repeatedly be switched between an on and off state. In CNI these advanced light microscopic techniques are mainly used to discover colocalizations within the molecular machinery on both sides of synaptic contacts (see: Hradsky J., et al. 2013; Fidzinski P., et al. 2015; Mikhaylova, M., et al., 2018).
Figure: Immunocytochemical localisation of pre- and postsynaptic proteins Bassoon (red) and Homer (green) along dendrites (blue) of hippocampal cell cultures by confocal (LSM) and STED microscopy.Metal induced energy transfer (Weber, Zuschratter)
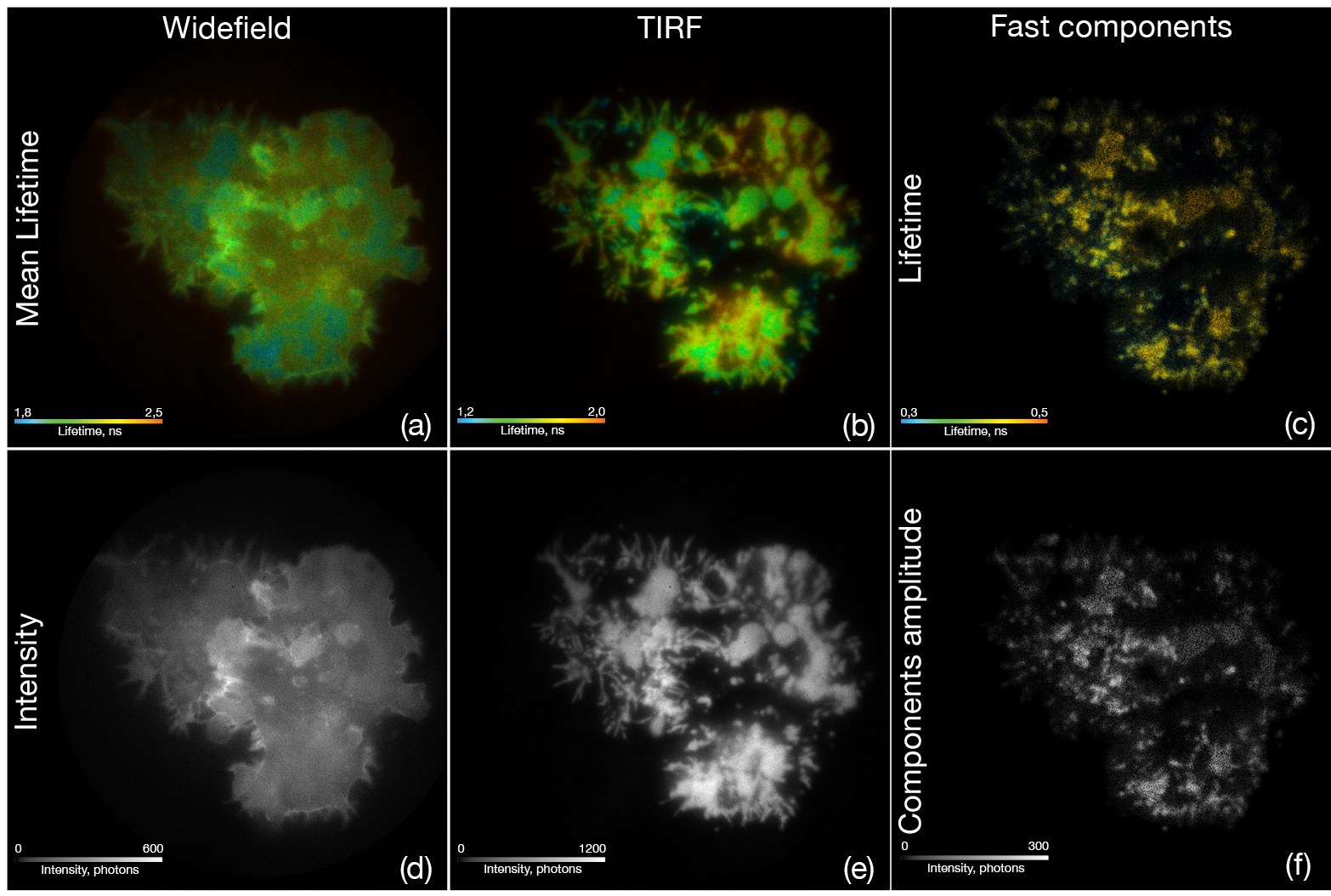
Metal induced energy transfer (MIET) is a nanoscopy technique to measure distances from fluorescent molecules to a metal film. Here we applied MIET acquisition using wide-field and TIRF fluorescence lifetime imaging microscopy (FLIM) in combination with a position sensitive single photon counting camera system (LINCam) to study T-cell receptor clustering within the plasma membrane of lymphocytes.
Figure: T-lymphocytes transfected with GFP-Lck were fixed on gold coated coverslips with a layer of CD3 antibodies. Under standard epifluorescence illumination (a, d) conditions the T-cells show the typical lifetime and intensity for GFP after excitation with a 488 nm pulsed laser. Switching to TIRF illumination (b, e) revealed a much higher lifetime contrast related to the MIET effect near the gold coating. Using a maximum entropy method (MEM) to discriminate fluorescence lifetime components, we show that the fast lifetime components of 0,38 ns and 0,82 ns form typical clustering of the protein kinase (c, f) along the plasma membrane after T-cell receptor stimulation by the CD3 antibodies.
Collaborators:- International and national: Y. Ma, K. Gauss, UNSW Sydney, J. Enderlein, Univ. Göttingen
- Magdeburg: Hartig, R., Kaestle, M., Müller, A., Philipsen, L., Schraven, B., Simeoni, L., (Med Fac. OvGU)
- LIN: Gundelfinger, E. D., Herrera-Molina, R., Thomas, U.
Funding:- DFG: ZU 59/10-2
- DFG SFB 854 TPZ 01
- EU CORBEL NETWORK: PID 2376
Lightsheet Microscopy (Kobler)
In addition to high-resolution microscopy 3D visualization of labeled structures in intact transparent tissue is a key topic of CNI. To this end, in cooperations with Dept. Genetics of Learning and Memory (B. Gerber, T. Saumweber), Dept. Neurochemistry and Molecular Biology (U. Thomas) and the Small Animal subunit of CNI (E. Budinger) we develop protocols for optimal clarification of Drosophila larvae and rodent brains and generate 3D records in the order of 50-400 GB by confocal or light-sheet microscopy.
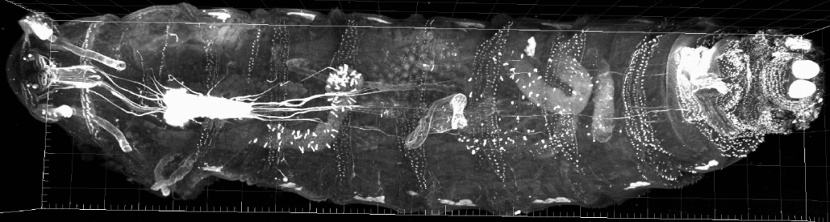
Figure: A whole Drosophila melanogaster L3 larva, expressing UAS-CAAX-mCherry pan neural, was cleared, sequentially imaged by focal sectioning and 3D reconstructed. The 38,5 GByte image consists of 52 tiles, each 707 z-slices. Cooperation with B. Gerber, T. Saumweber (Dept. Genetics of Learning and Memory, LIN)Electron Microscopy (Faber-Zuschratter, Stöter, Zuschratter)
Correlative super-resolution Light- and Electron Microscopy (CLEM), large scale electron microscopy as well as cryo-EM and 3D FIB-SEM are powerful tools to discover structural and functional changes at the ultrastructural level.
Within CNI we use CLEM and 3D FIB-SEM techniques to:
- analyse modifications in synaptic profiles of brain tissue or cell cultures
- explore the molecular organization and dynamics of the immune synapse (IS)
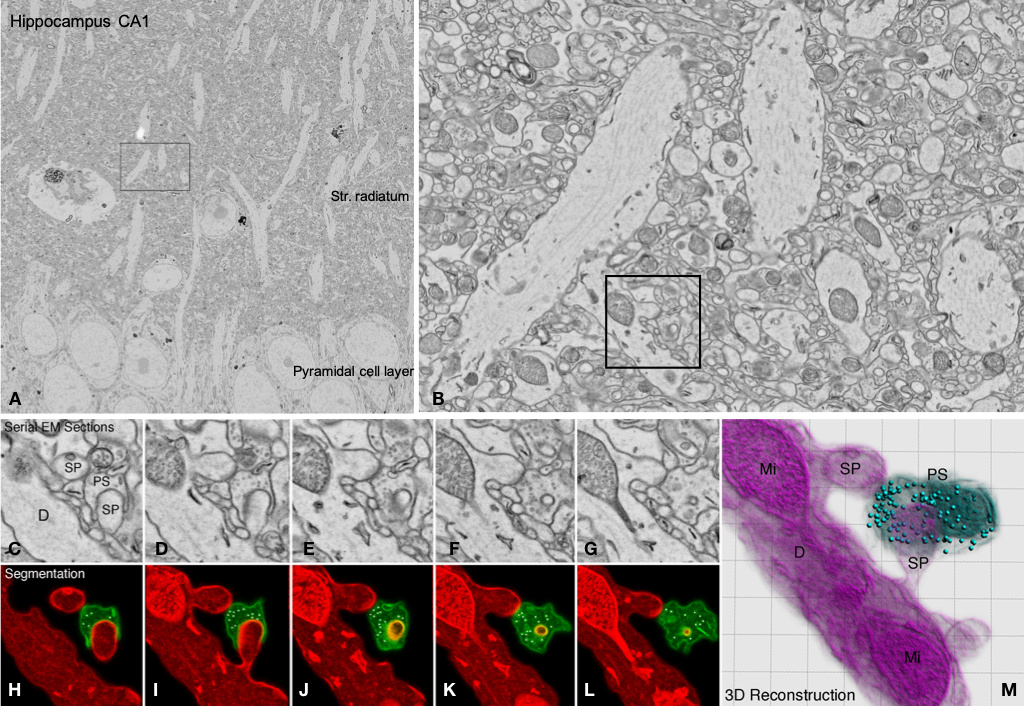
Figure: Overview and regions of interest from a series of ultrathin sections of hippocampal mouse brain. The images show some focal planes out of 21 serial 70 nm thick sections with a size of 500 MB each (total size of stack: 10,5 GB). A: overview; B: ROI from stratum radiatum in A; C-G: details of synaptic contacts along a dendrite of a pyramidal cell of CA1 shown in B, H-L: Segmentation of pre- and postsynaptic elements; M: 3D reconstruction from H-L.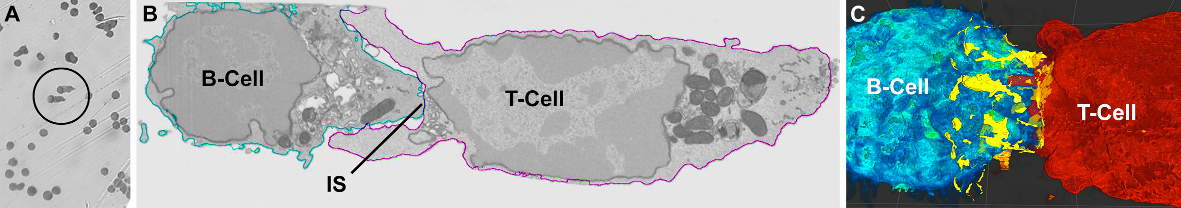
Figure: Correlated Light- (A) and Electron-microscopic view (B) of identified B-/T-cell pairs. EM-micrographs were taken by a scanning EM equipped with a focused ion beam (FIB) that allowed acquisition of serial images with an isotropic voxel size of 5 nm through the immune cells. (C) Contact points (yellow) along the immune synapse between B-T-cell membranes were identified and 3D reconstructed.
Since CNI doesn't own such instrumentation the necessary imaging techniques (e.g. 3D scanning electron microscope (SEM)) are provided by several collaborating facilities at Univ. Zürich, Univ. Utrecht and EMBL Heidelberg. The latter two belong to the European CORBEL network, which provides shared services for life sciences and grants travel funds to members of CNI.Collaborators:
- International and national: Bulitta, B., Jäntsch, L. (HZI Braunschweig), Lindenau, J. (Carl Zeiss Microscopy, Oberkochen), Ronchi, P., Schwab, Y., (EMBL Heidelberg), Kaech, A., Mateos, J., Ziegler, U. (University Zürich), Liv, N., Klumperman, J., (Univ. Med. Center Utrecht)
- Magdeburg: Hartig, R., Kaestle, M., Müller, A., Philipsen, L., Schraven, B., Simeoni, L., (Med Fac. OvGU)
- LIN: Gundelfinger, E. D., Herrera-Molina, R., Thomas, U.
Funding:- DFG: ZU 59/10-2
- DFG SFB 854 TPZ 01
- EU CORBEL NETWORK: PID 2376
- Dynamics of Learning & Cognition
Neural, psychophysiological and behavioral dynamics of learning by feedback (Lommerzheim, Wolff, Angenstein, Stadler, Brechmann)
Tutorial systems in a learning environment should support the user in reaching his specific leaning goals and react to his level of knowledge and individual abilities in a pedagogical reasonable manner. For this a comprehensive model of the user is essential taking the user’s prior knowledge and interaction history into account as well as his current affective state. Our research project will focus on an interactive learning task, in which the user is supported by a tutorial system component. Neurophysiological data (fMRT, EEG) and psychophysiological data (ECG, skin conductance, respiration) as well as details of user’s behavior (dynamics of key stroke, facial expressions) are recorded synchronously, in order to determine changing affective and cognitive user states by multimodal data analysis. The interdisciplinary analysis and interpretation of the interconnection between the partially weak signals of the single modalities, and the development and optimization of the classifiers for affect recognition are the primary goals of our research project.
Collaborators:
- Friedhelm Schwenker, Institute of Neural Information Processing, Ulm University
Funding:- DFG BR 2267/9-1 "Multimodal recognition of affect over the course of a tutorial learning experiment"
Brain mechanisms of information processing in dialogues (Wolff, Angenstein, Brechmann)
Humans interpret interactions with technical systems as a dialogue. Each action of the system represents a feedback on the previous action of the user, who may utilize it for goal-oriented actions in subsequent interaction steps and interprets it with regard to a potential intentionality of the system. Neuroscientifically, these dynamic processes and their interplay with mutual adaptation of behavior and the derivation of intentions and goals of the counterpart have hardly been investigated. The subproject deals with the question which neurobiological mechanisms to consider when conceptualizing anticipatory assistive systems. The aim is to record the temporal dynamics of the neuronal activity of dialog-relevant brain systems as well as psychophysiological and behavioral parameters during the course of interactive problem solving. From this, hypotheses about current strategies and intentions of the user are derived, which serve as a basis for tailor-made interventions and metadialogs initiated by technical systems. Understanding the effects of such dialogical interventions on brain activity and behavior will contribute to the neuroscientific foundation of human-computer interactions.
Collaborators:
- Myra Spiliopoulou, Knowledge Management & Discovery Lab, OVGU Magdeburg
Funding:- EFRE ZS/2017/10/88785 "Brain mechanisms of information processing in dialogues"
Effect of aging on lateralization of auditory processing and hemispheric interaction (Stadler, Brechmann, Angenstein)
The goal of this project is to better understand the deficits in central auditory processing in individuals with hearing impairment. Speech processing requires the processing of various basic acoustic parameters. The left and right auditory cortex are involved to different degrees in these processes. This different lateralization of processing requires efficient cooperation of the auditory cortices of both hemispheres during auditory processing. There is evidence that the lateralization of processing in the brain and the interaction between the hemispheres changes with age which may cause hearing deficits. We investigate how hemispheric involvement and hemispheric interaction in audition changes with age and what effect this has on auditory cognitive abilities. We want to develop intervention approaches to improve hemispheric interaction in old adults on an individual level to enable good listening competence.
Funding:
- DFG / AN 861/4-2 "Hemispheric interaction during lateralized auditory processing in humans: effects of task difficulty, training and age"
Lateralized auditory processing in cochlear implant users (Seidel, Stadler, Deliano, Angenstein)
Many people with severe hearing loss can be helped to hear well with cochlear implants. However, there are significant differences in speech perception and hearing quality between users after implantation. We investigate the relationships between implantation, lateralisation of auditory processing and hemispheric interaction in cochlear implant users. The aim is to improve the speech competence of cochlear implant users by taking into account the knowledge of the central processing of basic acoustic parameters of each individual user at each level of clinical care. The reasons for the significant differences in speech perception and hearing quality after cochlear implantation will be revealed. This should allow for an adjustment of the treatment to achieve the best possible hearing quality and speech competence after implantation.
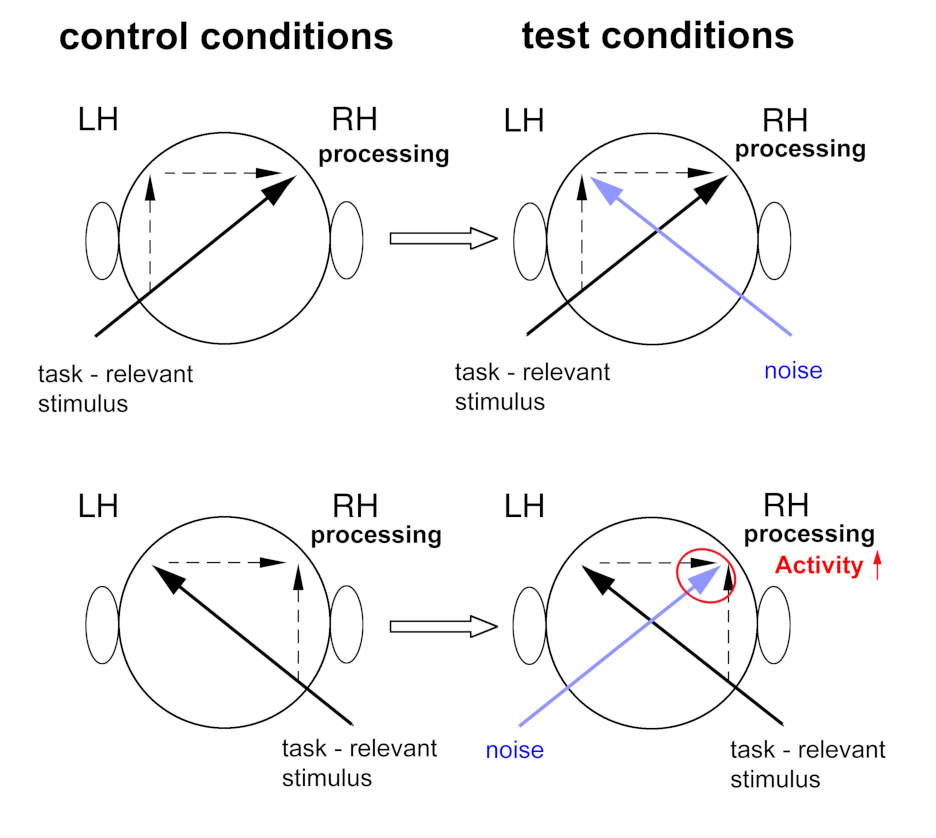 Figure: The contralateral noise procedure to investigate lateralized processing in the human auditory cortex and hemispheric interaction with fMRI
Figure: The contralateral noise procedure to investigate lateralized processing in the human auditory cortex and hemispheric interaction with fMRI
Collaborators:- Beate Wendt, Department of Otorhinolaryngology of the University Hospital of the Otto-von-Guericke-University Magdeburg; Director: Prof. Dr. med. Christoph Arens
- Jesko L. Verhey, Otto von Guericke University Magdeburg, Department of Experimental Audiology
- Horst Hessel, Cochlear Deutschland GmbH & Co. KG
Neural basis of program comprehension (Peitek, Brechmann)
Software developers spend most of their time with reading and understanding source code. Early theories of program comprehension have proposed hypothesis-driven (top-down) mechanisms and line-by-line (bottom-up) mechanisms of understanding code. However, the underlying cognitive processes of top-down and bottom-up program comprehension are still essentially unclear.
In the first project phase, we have set out to directly studying these processes by means of objective measures obtained from functional magnetic resonance imaging (fMRI). We have identified a set of brain regions specifically involved when participants are trying to understand source code. The pattern of activation was indicative of semantic processing tied to the left, i.e., the speech hemisphere, as well as attention and working memory functions. Promoting top-down comprehension, e.g., by informative beacons, have led to reduced activation in some of these brain areas, suggesting less cognitive effort, which we have confirmed by behavioral data. Our results have paved the way for other researchers using our neuro-imaging approach for follow-up studies on program comprehension.
The second project phase will refine our experimental framework by including eye tracking for specifying the time course of visual attention to inform a more specific analysis of the fMRI data as well as psycho-physiological measures (pupillometry, skin conductance, heart rate, and respiration) to identify changes in cognitive load. With this approach, we will study the impact of structural code elements (if-then-else statements, loops, recursion) as well as programming experience on top-down program comprehension and concomitant objective measures of brain activity, psycho-physiology, and behavior. Finally, based on the progress in the neurosciences on the neural basis of object processing, we will tackle the long-standing question about differences between object-oriented and functional programming.
Please find publications, source code and further details on this project at brains-on-code.github.io.
Collaborators:
- Janet Siegmund, Technical University Chemnitz
- Sven Apel, Saarland University
- Chris Parnin, NC State University, Raleigh, North Carolina, USA
Funding:- DFG BR 2267/2-1 "Understanding Program Comprehension in the Neuro-Imaging Age”
- DFG BR 2267/7-2 "Linking Program Comprehension to Neural, Behavioral, and Psycho-Physiological Correlates"
The Combinatorial NeuroImaging Core Facility
- Team
Fahmida Akter PhD Student +49-391-6263-95431 Dr. Nicole Angenstein Scientist, PI +49-391-6263-92182 Renate Blobel-Lüer Technical Assistance Human Imaging +49-391-6263-92172 Dr. André Brechmann CNI Coordinator, PI +49-391-6263-92161 Prof. Dr. Eike Budinger Responsible for 9.4T MRI, PI +49-391-6263-95421 Dr. Matthias Deliano Responsible for Human EEG, PI +49-391-6263-92151 Dr. Hanna Edler Application Specialist CLSM/STED, Lightsheet +49-391-6263-93221 Andreas Fügner Electrical Engineer Human Imaging +49-391-6263-92191 Dr. Jürgen Goldschmidt Responsible for SPECT/CT, PI +49-391-6263-95421 Lisa-Marie Goncalves PhD Student +49-391-6263-93471 Tobias Gottschall Research Data and Software Engineer +49-391-6263-92121 Anna Groppe PhD Student +49-391-6263-92141 Dr. Hongbo Jia Responsible for 2 Photon Imaging +49-391-6263-93331 Dr. Oliver Kobler Responsible for CLSM/STED, Light Sheet +49-391-6263-93221 Anke Michalsky Technical Assistance 3T MRI +49-391-6263-92191 Dr. Norman Peitek Guest Scientist +49-391-6263-92152 Holger Reim Technical Assistance SPECT/CT +49-391-6263-95431 Gabriele Schöps Technical Assistance EEG +49-391-6263-91351 Dr. Jörg Stadler Responsible for Human MRI +49-391-6263-92171 Janet Stallmann Technical Assistance 9.4T MRI +49-391-6263-95461 Patricia Wenk Application Specialist 9.4T MRI +49-391-6263-95431 - Publications
Publications
2024
Angenstein N. 2024. Asymmetries and hemispheric interaction in the auditory system of elderly people. Frontiers in Neuroimaging. 2:Article 1320989. https://doi.org/10.3389/fnimg.2023.1320989
Murkar R., von Heckel C., Walles H., Moch T.B., Arens C., Davaris N., Weber A., Zuschratter W., Baumann S., Reinhardt J., Kopp S. 2024. Establishment of a Human Immunocompetent 3D Tissue Model to Enable the Long-Term Examination of Biofilm–Tissue Interactions. Bioengineering. 11 (2), art. no. 187. https://doi.org/10.3390/bioengineering11020187
Muehlberg F, Mohnike K, Grosser OS, Pech M, Goldschmidt J, Smalla K-H, Seidensticker R, Ümütlü MR, Deniz S, Ricke J, et al. 2024. In vivo evaluation of tumor uptake and bio-distribution of 99mTc-labeled 1-thio-β-D-glucose and 5-thio-D-glucose in mice model. EJNMMI radiopharmacy and chemistry. 9(1):Article 26. https://doi.org/10.1186/s41181-024-00253-3
Oelschlegel AM, Bhattacharjee R, Wenk P, Harit K, Rothkötter H-J, Koch SP, Boehm-Sturm P, Matuschewski K, Budinger E, Schlüter D, et al. 2024. Beyond the microcirculation: sequestration of infected red blood cells and reduced flow in large draining veins in experimental cerebral malaria. Nature Communications. 15(1):Article 2396. https://doi.org/10.1038/s41467-024-46617-w
2023
Adasme T, Hidalgo C, Herrera-Molina R. 2023. Editorial: Emerging views and players in neuronal calcium signaling: synaptic plasticity, learning/memory, aging and neuroinflammation. Frontiers in Cellular Neuroscience. 17:Article 1197417. https://doi.org/10.3389/fncel.2023.1197417
Angenstein N, Brancucci A. 2023. Editorial: Hemispheric asymmetries in the auditory domain, volume II. Frontiers in Neuroscience. 17:Article 1263317. https://doi.org/10.3389/fnins.2023.1263317
Bellmann P, Kessler V, Brechmann A, Schwenker F. 2023. Button Press Dynamics: Beyond Binary Information in Button Press Decisions. Lecture Notes in Networks and Systems: 469-477. https://doi.org/10.1007/978-981-19-0105-8_46
Grandjean J, Desrosiers-Gregoire G, Anckaerts C, Angeles-Valdez D, Ayad F, Barrière DA, Blockx I, Bortel A, Broadwater M, Cardoso BM, et al. 2023. A consensus protocol for functional connectivity analysis in the rat brain. Nature Neuroscience. 26(4):673-681. https://doi.org/10.1038/s41593-023-01286-8
Grochowska KM, Gomes GM, Raman R, Kaushik R, Sosulina L, Kaneko H, Oelschlegel AM, Yuanxiang P, Reyes-Resina I, Bayraktar G, … Goldschmidt J. …et al. 2023. Jacob-induced transcriptional inactivation of CREB promotes Aβ-induced synapse loss in Alzheimer's disease. The EMBO journal. 42(4):Article e112453. https://doi.org/10.15252/embj.2022112453
Gu M, Li X, Liang S, Zhu J, Sun P, He Y, Yu H, Li R, Zhou Z, Lyu J, … Jia H. … et al. 2023. Rabies virus-based labeling of layer 6 corticothalamic neurons for two-photon imaging in vivo. iScience. 26(5):Article 106625. https://doi.org/10.1016/j.isci.2023.106625
Huang J, Liang S, Li L, Li X, Liao X, Hu Q, Zhang C, Jia H, Chen X, Wang M, et al. 2023. Daily two-photon neuronal population imaging with targeted single-cell electrophysiology and subcellular imaging in auditory cortex of behaving mice. Frontiers in Cellular Neuroscience. 17:Article 1142267. https://doi.org/10.3389/fncel.2023.1142267
Huang W, Wang Y, Qin J, He C, Li Y, Wang Y, Li M, Lyu J, Zhou Z, Jia H, et al. 2023. A corticostriatal projection for sound-evoked and anticipatory motor behavior following temporal expectation. NeuroReport. 34(1):1-8. https://doi.org/10.1097/WNR.0000000000001851
Jamaludeen N, Kuhn F, Brechmann A, Fuhrmann F, Remy S, Spiliopoulou M. 2023. Inferring Salient Motifs during Learning Experiments. Sicilia R, Kane B, Almeida JR, Spiliopoulou M, Andrades JAB, Placidi G, Gonzalez AR, Hrsg. in Proceedings - 2023 IEEE 36th International Symposium on Computer-Based Medical Systems, CBMS 2023. IEEE. S. 245-251. (Proceedings - IEEE Symposium on Computer-Based Medical Systems). https://doi.org/10.1109/CBMS58004.2023.00225
Li R, Huang J, Li L, Zhao Z, Liang S, Liang S, Wang M, Liao X, Lyu J, Zhou Z, et al. 2023. Holistic bursting cells store long-term memory in auditory cortex. Nature Communications. 14(1):Article 8090. https://doi.org/10.1038/s41467-023-43620-5
Li X, Song S, Yao J, Liao X, Chen M, Zhai J, Lang L, Lin C, Zhang N, Yuan C, … Jia H … et al. 2023. Autofluorescence spectral analysis for detecting urinary stone composition in emulated intraoperative ambient. Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy. 300:Article 122913. https://doi.org/10.1016/j.saa.2023.122913
Li R, Wang S, Lyu J, Chen K, Sun X, Huang J, Sun P, Liang S, Li M, Yang M, … Jia H … et al. 2023. Ten-kilohertz two-photon microscopy imaging of single-cell dendritic activity and hemodynamics in vivo. Neurophotonics. 10(2):Article 025006. https://doi.org/10.1117/1.NPh.10.2.025006
Malci A, Lin X, Shi YS, Herrera-Molina R. 2023. Neuroplastin in Ca2+ signal regulation and plasticity of glutamatergic synapses. Neural Regeneration Research. 18(8):1705-1706. https://doi.org/10.4103/1673-5374.363826
Mancini N, Thoener J, Tafani E, Pauls D, Mayseless O, Strauch M, Eichler K, Champion A, Kobler O, Weber D, et al. 2023. Rewarding capacity of optogenetically activating a giant GABAergic central-brain interneuron in larval Drosophila. Journal of Neuroscience. 43(44):7393-7428. https://doi.org/10.1523/JNEUROSCI.2310-22.2023
Montag D, Liang Y, Herrera-Molina R, Lin X, Ormazabal-Toledo R, Yao S, Shi YS. 2023. Deafness causing neuroplastin missense variants fail to promote plasma membrane Ca2+-ATPase levels and Ca2+ transient regulation in brain neurons. Journal of Biological Chemistry.
Nöthen T, Sarabi MA, Weinert S, Zuschratter W, Morgenroth R, Mertens PR, Braun-Dullaeus RC, Medunjanin S. 2023. DNA-Dependent Protein Kinase Mediates YB-1 (Y-Box Binding Protein)-Induced Double Strand Break Repair. Arteriosclerosis, thrombosis, and vascular biology. 43(2):300-311. https://doi.org/10.1161/ATVBAHA.122.317922
Sen ZD, Chand T, Danyeli LV, Kumar VJ, Colic L, Li M, Yemisken M, Javaheripour N, Refisch A, Opel N, et al. 2023. The effect of ketamine on affective modulation of the startle reflex and its resting-state brain correlates. Scientific Reports. 13(1):Article 13323. https://doi.org/10.1038/s41598-023-40099-4
Stadler J, Brechmann A, Angenstein N. 2023. Effect of age on lateralized auditory processing. Hearing Research. 434:Article 108791. https://doi.org/10.1016/j.heares.2023.108791
Stollmeier M, Kahlert S, Zuschratter W, Oster M, Wimmers K, Isermann B, Rothkötter H-J, Nossol C. 2023. Air-liquid interface cultures trigger a metabolic shift in intestinal epithelial cells (IPEC-1). Histochemistry and cell biology. https://doi.org/10.1007/s00418-023-02180-x
Stöter T, Gottschall T, Schrader A, Zentis P, Valencia-Schneider M, Kandpal N, Zuschratter W, Schauss A, Dickscheid T, Mühlhaus T, et al. 2023. Combining the BIDS and ARC Directory Structures for Multimodal Research Data Organization. https://doi.org/10.5281/zenodo.8349563
Thane M, Paisios E, Stöter T, Krüger A-R, Gläß S, Dahse A-K, Scholz N, Gerber B, Lehmann DJ, Schleyer M. 2023. High-resolution analysis of individual Drosophila melanogaster larvae uncovers individual variability in locomotion and its neurogenetic modulation. Open biology. 13(4):Article 220308. https://doi.org/10.1098/rsob.220308
Vosskuhl J, Herrmann CS, Brechmann A, Scheich H. 2023 Simultaneous Electroencephalography and Functional Magnetic Resonance Imaging of the Human Auditory System. In EEG-fMRI: Physiological Basis, Technique, and Applications, Second Edition, pp. 547-564. https://doi.org/10.1007/978-3-031-07121-8_22
Wolff S, Brechmann A. 2023. Dorsal posterior cingulate cortex responds to negative feedback information supporting learning and relearning of response policies. Cerebral Cortex. 33(10):5947-5956. https://doi.org/10.1093/cercor/bhac473
2022
Brancucci A, Angenstein N. 2022. Editorial: Hemispheric Asymmetries in the Auditory Domain. Frontiers in Behavioral Neuroscience. 16:Article 892786. https://doi.org/10.3389/fnbeh.2022.892786
Chander BS, Deliano M, Azañón E, Büntjen L, Stenner M-P. 2022. Non-invasive recording of high-frequency signals from the human spinal cord. NeuroImage. 253:Article 119050. https://doi.org/10.1016/j.neuroimage.2022.119050
Deliano M, Seidel P, Vorwerk U, Stadler B, Angenstein N. 2022. Effect of cochlear implant side on early speech processing in adults with single-sided deafness. Clinical Neurophysiology. 140:29-39. https://doi.org/10.1016/j.clinph.2022.05.008
Ding F, Liang S, Li R, Yang Z, He Y, Yang S, Duan Q, Zhang J, Lyu J, Zhou Z, et al. 2022. Astrocytes exhibit diverse Ca2+ changes at subcellular domains during brain aging. Frontiers in Aging Neuroscience. 14:Article 1029533. https://doi.org/10.3389/fnagi.2022.1029533
Guzmán Salas S, Weber A, Malci A, Lin X, Herrera-Molina R, Cerpa W, Dorador C, Signorelli J, Zamorano P. 2022. The metabolite p-cresol impairs dendritic development, synaptogenesis and synapse function in hippocampal neurons: Implications for autism spectrum disorder. Journal of Neurochemistry. https://doi.org/10.1111/jnc.15604
Jamaludeen N, Unnikrishnan V, Brechmann A, Spiliopoulou M. 2022. Discovering Instantaneous Granger Causalities in Non-stationary Categorical Time Series Data. Michalowski M, Abidi SSR, Abidi S, Hrsg. in Artificial Intelligence in Medicine - 20th International Conference on Artificial Intelligence in Medicine, AIME 2022, Proceedings. Springer Lecture Notes in Computer Science: 200-209. https://doi.org/10.1007/978-3-031-09342-5_19
Malci A, Sandoval R, Gundelfinger ED, Naumann M, Seidenbecher C, Herrera-Molina R. 2022. Ca2+ signaling in postsynaptic neurons: neuroplastin-65 regulates the interplay between plasma membrane Ca2+ ATPases and ionotropic glutamate receptors. Cell calcium. 106:Article 102623. https://doi.org/10.1016/j.ceca.2022.102623
Meka DP, Kobler O, Hong S, Friedrich CM, Wuesthoff S, Henis M, Schwanke B, Krisp C, Schmuelling N, Rueter R, Ruecker T, Betleja E, Cheng T, Mahjoub MR, Soba P, Schlüter H, Fornasiero EF, Calderon de Anda F. 2022. Centrosome-dependent microtubule modifications set the conditions for axon formation. Cell Reports. 39(3):Article 110686. https://doi.org/10.1016/j.celrep.2022.110686
Oleksiievets N, Mathew C, Thiele JC, Gallea JI, Nevskyi O, Gregor I, Weber A, Tsukanov R, Enderlein J. 2022. Single-Molecule Fluorescence Lifetime Imaging Using Wide-Field and Confocal-Laser Scanning Microscopy: A Comparative Analysis. Nano letters. https://doi.org/10.1021/acs.nanolett.2c01586
Qin H, Fu L, Jian T, Jin W, Liang M, Li J, Chen Q, Yang X, Du H, Liao X, et al. 2022. REM sleep-active hypothalamic neurons may contribute to hippocampal social-memory consolidation. Neuron. 110(23):4000-4014.e6. https://doi.org/10.1016/j.neuron.2022.09.004
Saldeitis K, Jeschke M, Michalek A, Henschke JU, Wetzel W, Ohl FW, Budinger E. 2022. Selective interruption of auditory interhemispheric crosstalk impairs discrimination learning of frequency-modulated tone direction but not gap detection and discrimination. Journal of Neuroscience. 42(10):2025-2038. https://doi.org/10.1523/JNEUROSCI.0216-21.2022
Tang J, Xue R, Wang Y, Li M, Jia H, Pakan JMP, Li L, Chen X, Li X. 2022. Optical Fiber-Based Recording of Climbing Fiber Ca2+ Signals in Freely Behaving Mice. Biology. 11(6):Article 907. https://doi.org/10.3390/biology11060907
Tegelbeckers J, Brechmann A, Breitling-Ziegler C, Bonath B, Flechtner H-H, Krauel K. 2022. Neural Mechanisms Underlying the Effects of Novel Sounds on Task Performance in Children With and Without ADHD. Frontiers in Human Neuroscience. 16:Article 878994. https://doi.org/10.3389/ fnhum.2022.878994
Wackernagel L-M, Abdi Sarabi M, Weinert S, Zuschratter W, Richter K, Fischer KD, Braun-Dullaeus RC, Medunjanin S. 2022. IKKγ/NEMO Localization into Multivesicular Bodies. International Journal of Molecular Sciences. 23(12):Article 6778. https://doi.org/10.3390/ijms23126778
Wang M, Liu K, Pan J, Li J, Sun P, Zhang Y, Li L, Guo W, Xin Q, Zhao Z, Liu Y, Zhou Z, Lyu J, Zheng T, Han Y, Zhang C, Liao X, Zeng S, Jia H, Chen X. 2022. Brain-wide projection reconstruction of single functionally defined neurons. Nature Communications. 13(1):Article 1531. doi.org/10.1038/s41467-022-29229-0
Wolff S, Brechmann A. 2022. Dorsal posterior cingulate cortex responds to negative feedback information supporting learning and relearning of response policies. Cerebral Cortex. https://doi.org/10.1093/cercor/bhac473
2021
Braun K, Mannewitz A, Bock J, Kreitz S, Hess A, Scheich H, Goldschmidt J. 2021. Imaging of Functional Brain Circuits during Acquisition and Memory Retrieval in an Aversive Feedback Learning Task: Single Photon Emission Computed Tomography of Regional Cerebral Blood Flow in Freely Behaving Rats. Brain Sciences. 11(5):Article 659. https://doi.org/10.3390/brainsci11050659
Buentjen L, Vicheva P, Chander BS, Beccard S-A, Coutts C, Azañón E, Stenner M-P, Deliano M. 2021. Spatial Filtering of Electroencephalography Reduces Artifacts and Enhances Signals Related to Spinal Cord Stimulation (SCS). Neuromodulation : journal of the International Neuromodulation Society. 24(8):1317-1326. https://doi.org/10.1111/ner.13266
Debska-Vielhaber G, Miller I, Peeva V, Zuschratter W, Walczak J, Schreiber S, Petri S, Machts J, Vogt S, Szczepanowska J, Gellerich FN, Hermann A, Vielhaber S, Kunz WS. 2021. Impairment of mitochondrial oxidative phosphorylation in skin fibroblasts of SALS and FALS patients is rescued by in vitro treatment with ROS scavengers. Experimental Neurology. 339:Article 113620. https://doi.org/10.1016/j.expneurol.2021.113620
Düsedau HP, Steffen J, Figueiredo CA, Boehme JD, Schultz K, Erck C, Korte M, Faber-Zuschratter H, Smalla K-H, Dieterich D, Kröger A, Bruder D, Dunay IR. 2021. Influenza A Virus (H1N1) Infection Induces Microglial Activation and Temporal Dysbalance in Glutamatergic Synaptic Transmission. mBio. 12(5):Article e0177621. https://doi.org/10.1128/mBio.01776-21
El-Tabbal M, Niekisch H, Henschke JU, Budinger E, Frischknecht R, Deliano M, Happel MFK. 2021. The extracellular matrix regulates cortical layer dynamics and cross-columnar frequency integration in the auditory cortex. Communications biology. 4(1):Article 322. https://doi.org/10.1038/s42003-021-01837-4
Hajizadeh A, Matysiak A, Brechmann A, König R, May PJC. 2021. Why do humans have unique auditory event-related fields? Evidence from computational modeling and MEG experiments. Psychophysiology. 58(4):Article e13769. https://doi.org/10.1111/psyp.13769
Ilic K, Lin X, Malci A, Stojanović M, Puljko B, Rožman M, Vukelić Ž, Heffer M, Montag D, Schnaar RL, Kalanj-Bognar S, Herrera-Molina R, Mlinac-Jerkovic K. 2021. Plasma membrane calcium ATPase-neuroplastin complexes are selectively stabilized in GM1-containing lipid rafts. International Journal of Molecular Sciences. 22(24):Article 13590. https://doi.org/10.3390/ijms222413590
Kobler O, Weiglein A, Hartung K, Chen Y-C, Gerber B, Thomas U. 2021. A quick and versatile protocol for the 3D visualization of transgene expression across the whole body of larval Drosophila. Journal of Neurogenetics. 35(3):306-319. https://doi.org/10.1080/01677063.2021.1892096
Krick N, Ryglewski S, Pichler A, Bikbaev A, Götz T, Kobler O, Heine M, Thomas U, Duch C. 2021. Separation of presynaptic Cav2 and Cav1 channel function in synaptic vesicle exo- and endocytosis by the membrane anchored Ca2+ pump PMCA. Proceedings of the National Academy of Sciences of the United States of America. 118(28):Article e2106621118. https://doi.org/10.1073/pnas.2106621118
Lin X, Brunk MGK, Yuanxiang P, Curran AW, Zhang E, Stöber F, Goldschmidt J, Gundelfinger ED, Vollmer M, Happel MFK, Herrera-Molina R, Montag D. 2021. Neuroplastin expression is essential for hearing and hair cell PMCA expression. Brain Structure and Function. 226(5):1533-1551. https://doi.org/10.1007/s00429-021-02269-w
Lin X, Liang Y, Herrera-Molina R, Montag D. 2021. Neuroplastin in Neuropsychiatric Diseases. Genes. 12(10):Article 1507. https://doi.org/10.3390/genes12101507
Peitek N, Apel S, Parnin C, Brechmann A, Siegmund J. 2021. Program Comprehension and Code Complexity Metrics: An fMRI Study. In International Conference on Software Engineering (ICSE). IEEE Computer Society. pp. 524-536. https://doi.org/10.1109/ICSE43902.2021.00056
Prior MJW, Bast T, McGarrity S, Goldschmidt J, Vincenz D, Seaton A, Hall G, Pitiot A. 2021. Ratlas-LH: An MRI template of the Lister hooded rat brain with stereotaxic coordinates for neurosurgical implantations. Brain and neuroscience advances. 5:Article 23982128211036332. https://doi.org/10.1177/23982128211036332
Saldeitis K, Jeschke M, Budinger E, Ohl FW, Happel MFK. 2021. Laser-Induced Apoptosis of Corticothalamic Neurons in Layer VI of Auditory Cortex Impact on Cortical Frequency Processing. Frontiers in neural circuits. 15:Article 659280. https://doi.org/10.3389/fncir.2021.659280
Wang M, Weber A, Hartig R, Zheng Y, Krafft D, Vidaković-Koch T, Zuschratter W, Ivanov I, Sundmacher K. 2021. Scale up of Transmembrane NADH Oxidation in Synthetic Giant Vesicles. Bioconjugate chemistry. 32(5):897-903. https://doi.org/10.1021/acs.bioconjchem.1c00096
Wendt B, Stadler J, Verhey JL, Hessel H, Angenstein N. 2021. Effect of contralateral noise on speech intelligibility. Neuroscience. 459:59-69. https://doi.org/10.1016/j.neuroscience.2021.01.034
2020
Abolfazli A, Brechmann A, Wolff S, Spiliopoulou M. 2020. Machine learning identifies the dynamics and influencing factors in an auditory category learning experiment. Scientific Reports. 10(1):Article 6548. https://doi.org/10.1038/s41598-020-61703-x
Aggelopoulos NC, Deike S, Selezneva E, Scheich H, Brechmann A, Brosch M. 2020. Predictive cues for auditory stream formation in humans and monkeys. European Journal of Neuroscience. 51(5):1254-1264. https://doi.org/10.1111/ejn.13808
Buentjen L, Vicheva P, Chander BS, Beccard S-A, Coutts C, Azañón E, Stenner M-P, Deliano M. 2020. Spatial Filtering of Electroencephalography Reduces Artifacts and Enhances Signals Related to Spinal Cord Stimulation (SCS). Neuromodulation: Journal of the International Neuromodulation Society. https://doi.org/10.1111/ner.13266
Deane KE, Brunk MGK, Curran AW, Zempeltzi MM, Ma J, Lin X, Abela F, Aksit S, Deliano M, Ohl FW, Happel MFK. 2020. Ketamine anaesthesia induces gain enhancement via recurrent excitation in granular input layers of the auditory cortex. Journal of Physiology. https://doi.org/10.1113/JP279705
Deliano M, Brunk MGK, El-Tabbal M, Zempeltzi MM, Happel MFK, Ohl FW. 2020. Dopaminergic neuromodulation of high gamma stimulus phase-locking in gerbil primary auditory cortex mediated by D1/D5-receptors. European Journal of Neuroscience. 51(5):1315-1327. https://doi.org/10.1111/ejn.13898
Dürschmid S, Reichert C, Walter N, Hinrichs H, Heinze H-J, Ohl FW, Tononi G, Deliano M. 2020. Self-regulated critical brain dynamics originate from high frequency-band activity in the MEG. PloS one. 15 (6):e0233589. https://doi.org/10.1371/journal.pone.0233589
Goldschmidt J, Oelschlegel A. 2020. Functional Neuroimaging in Rodents Using Cerebral Blood Flow SPECT. Frontiers in Physics. 8:Article 152. https://doi.org/10.3389/fphy.2020.00152
Hanke M, Mathôt S, Ort E, Peitek N, Stadler J, Wagner A. 2020. A Practical Guide to Functional Magnetic Resonance Imaging with Simultaneous Eye Tracking for Cognitive Neuroimaging Research. Pollmann S, editor. In Neuromethods: Spatial Learning and Attention Guidance. Humana Press. pp. 291-305. (Neuromethods). https://doi.org/10.1007/7657_2019_31
Heimrath K, Brechmann A, Blobel-Lüer R, Stadler J, Budinger E, Zaehle, T. 2020. Transcranial direct current stimulation (tDCS) over the auditorymcortex modulates GABA and glutamate: a 7 T MR-spectroscopy study.
Scientific Reports, 10 (1), art. no. 20111. https://doi.org/10.1038/s41598-020-77111-0Lommerzheim M, Prezenski S, Russwinkel N, Brechmann A. 2020. Category learning as a use case for anticipating individual human decision making by intelligent systems. Advances in Intelligent Systems and Computing, 1131 AISC, pp. 159-164. https://doi.org/10.1007/978-3-030-39512-4_25
Oleksiievets N, Thiele JC, Weber A, Gregor I, Nevskyi O, Isbaner S, Tsukanov R, Enderlein J. 2020. Wide-Field Fluorescence Lifetime Imaging of Single Molecules. Journal of Physical Chemistry A. 124(17):3494-3500. https://doi.org/10.1021/acs.jpca.0c01513
Medunjanin S, Putzier M, Nöthen T, Weinert S, Kähne T, Luani B, Zuschratter W, Braun-Dullaeus RC. 2020. DNA-PK: gatekeeper for IKKγ/NEMO nucleocytoplasmic shuttling in genotoxic stress-induced NF-kappaB activation. Cellular and Molecular Life Sciences. https://doi.org/10.1007/s00018-019-03411-y
Park JY, Polzehl J, Chatterjee S, Brechmann A, Fiecas M. 2020. Semiparametric modeling of time-varying activation and connectivity in task-based fMRI data. Computational Statistics and Data Analysis, 150, art. no. 107006 https://doi.org/10.1016/j.csda.2020.107006
Peitek N, Siegmund J, Apel S, Kastner C, Parnin C, Bethmann A, Leich T, Saake G, Brechmann A. 2020. A Look into Programmers Heads. IEEE Transactions on Software Engineering. 46(4):442-462. https://doi.org/10.1109/TSE.2018.2863303
Siegmund J, Peitek N, Brechmann A, Parnin C, Apel S. 2020. Studying Programming in the Neuroage: Just a Crazy Idea?. Communications of the ACM. 63(6):30-34. https://doi.org/10.1145/3347093
Sikka A, Jamalabadi H, Krylova M, Alizadeh S, van der Meer JN, Danyeli L, Deliano M, Vicheva P, Hahn T, Koenig T, Bathula DR, Walter M. 2020. Investigating the temporal dynamics of electroencephalogram (EEG) microstates using recurrent neural networks. Human Brain Mapping. 41(9):2334-2346. https://doi.org/10.1002/hbm.24949
Vemula SK, Malci A, Junge L, Lehmann AC, Rama R, Hradsky J, Matute RA, Weber A, Prigge M, Naumann M, Kreutz MR, Seidenbecher CI, Gundelfinger ED, Herrera-Molina R. 2020. The Interaction of TRAF6 With Neuroplastin Promotes Spinogenesis During Early Neuronal Development. Frontiers in Cell and Developmental Biology. 8:Article 579513. https://doi.org/10.3389/fcell.2020.579513
Weber A, Zuschratter W, Hauser MJB. 2020. Partial synchronisation of glycolytic oscillations in yeast cell populations. Scientific Reports. 10(1):Article 19714. https://doi.org/10.1038/s41598-020-76242-8
Weidner TC, Vincenz D, Brocka M, Tegtmeier J, Oelschlegel AM, Ohl FW, Goldschmidt J, Lippert MT. 2020. Matching stimulation paradigms resolve apparent differences between optogenetic and electrical VTA stimulation. Brain Stimulation. 13(2):363-371. https://doi.org/10.1016/j.brs.2019.11.005
Wolff S, Kohrs C, Angenstein N, Brechmann A. 2020. Dorsal posterior cingulate cortex encodes the informational value of feedback in human-computer interaction. Scientific Reports. 10(1):Article 13030. https://doi.org/10.1038/s41598-020-68300-y
Zempeltzi MM, Kisse M, Brunk MGK, Glemser C, Aksit S, Deane KE, Maurya S, Schneider L, Ohl FW, Deliano M, Happel MFK. 2020. Task rule and choice are reflected by layer-specific processing in rodent auditory cortical microcircuits. Communications biology. 3(1):Article 345. https://doi.org/10.1038/s42003-020-1073-3
2019
Angenstein F. 2019. The role of ongoing neuronal activity for baseline and stimulus-induced BOLD signals in the rat hippocampus. NeuroImage. 202:Article 116082. https://doi.org/10.1016/j.neuroimage.2019.116082
Bauer J, Siegmund J, Peitek N, Hofmeister J, Apel S. 2019. Indentation: Simply a Matter of Style or Support for Program Comprehension?. In Proceedings - 2019 IEEE/ACM 27th International Conference on Program Comprehension, ICPC 2019. IEEE. pp. 154-164. (IEEE International Conference on Program Comprehension). https://doi.org/10.1109/ICPC.2019.00033
Bovet-Carmona M, Krautwald K, Menigoz A, Vennekens R, Balschun D, Angenstein F. 2019. Low frequency pulse stimulation of Schaffer collaterals in Trpm4−/− knockout rats differently affects baseline BOLD signals in target regions of the right hippocampus but not BOLD responses at the site of stimulation. NeuroImage. 188:347-356. https://doi.org/10.1016/j.neuroimage.2018.12.020
Brechmann A, Angenstein N. 2019. The impact of task difficulty on the lateralization of processing in the human auditory cortex. Human Brain Mapping. 40(18):5341-5353. https://doi.org/10.1002/hbm.24776
Brunk MGK, Deane KE, Kisse M, Deliano M, Vieweg S, Ohl FW, Lippert MT, Happel MFK. 2019. Optogenetic stimulation of the VTA modulates a frequency-specific gain of thalamocortical inputs in infragranular layers of the auditory cortex. Scientific Reports. 9(1):Article 20385. https://doi.org/10.1038/s41598-019-56926-6
Döring M, Blees H, Koller N, Tischer-Zimmermann S, Müsken M, Henrich F, Becker J, Grabski E, Wang J, Janssen H, Zuschratter W, Neefjes J, Klawonn F, Eiz-Vesper B, Tampé R, Kalinke U. 2019. Modulation of TAP-dependent antigen compartmentalization during human monocyte-to-DC differentiation. Blood advances. 3(6):839-850. https://doi.org/10.1182/bloodadvances.2018027268
Leschik J, Eckenstaler R, Endres T, Munsch T, Edelmann E, Richter K, Kobler O, Fischer K-D, Zuschratter W, Brigadski T, Lutz B, Lessmann V. 2019. Prominent Postsynaptic and Dendritic Exocytosis of Endogenous BDNF Vesicles in BDNF-GFP Knock-in Mice. Molecular Neurobiology. 56(10):6833-6855. https://doi.org/10.1007/s12035-019-1551-0
Macharadze T, Budinger E, Brosch M, Scheich H, Ohl FW, Henschke JU. 2019. Early Sensory Loss Alters the Dendritic Branching and Spine Density of Supragranular Pyramidal Neurons in Rodent Primary Sensory Cortices. Frontiers in neural circuits. 13:Article 61. https://doi.org/10.3389/fncir.2019.00061
Mattern H, Sciarra A, Lüsebrink F, Acosta-Cabronero J, Speck O. 2019. Prospective motion correction improves high-resolution quantitative susceptibility mapping at 7T. Magnetic Resonance in Medicine. 81(3):1605-1619. https://doi.org/10.1002/mrm.27509
Meka DP, Scharrenberg R, Zhao B, Kobler O, König T, Schaefer I, Schwanke B, Klykov S, Richter M, Eggert D, Windhorst S, Dotti CG, Kreutz MR, Mikhaylova M, Calderon de Anda F. 2019. Radial somatic F-actin organization affects growth cone dynamics during early neuronal development. EMBO Reports. 20(12):Article e47743. https://doi.org/10.15252/embr.201947743
Peitek N, Apel S, Brechmann A, Parnin C, Siegmund J. 2019. CodersMUSE: Multi-Modal Data Exploration of Program-Comprehension Experiments. In Proceedings - 2019 IEEE/ACM 27th International Conference on Program Comprehension, ICPC 2019. IEEE. pp. 126-129. https://doi.org/10.1109/ICPC.2019.00027
Saldeitis K, Richter K, Fischer K-D, Ohl FW, Mateos JM, Budinger E. 2019. Ultrastructure of giant thalamic terminals in the auditory cortex. European Journal of Neuroscience. 50(9):3445-3453. https://doi.org/10.1111/ejn.14509
Schicknick H, Henschke JU, Budinger E, Ohl FW, Gundelfinger ED, Tischmeyer W. 2019. β-adrenergic modulation of discrimination learning and memory in the auditory cortex. European Journal of Neuroscience. 50(7):3141-3163. https://doi.org/10.1111/ejn.14480
van Bommel B, Konietzny A, Kobler O, Bär J, Mikhaylova M. 2019. F-actin patches associated with glutamatergic synapses control positioning of dendritic lysosomes. EMBO Journal. 38(15):e101183. https://doi.org/10.15252/embj.2018101183
Wagner M, Mahlmann A, Deindl E, Zuschratter W, Riek-Burchardt M, Kostin S, Luani B, Baer C, Youssef A, Herold J. 2019. Clinical improvement and enhanced collateral vessel growth after xenogenic monocyte transplantation. American journal of translational research. 11(7):4063-4076.
Wendemuth A, Boeck R, Nuernberger A, Al-Hamadi A, Brechmann A, Ohl FW. 2019. Intention-Based Anticipatory Interactive Systems. In Proceedings - 2018 IEEE International Conference on Systems, Man, and Cybernetics, SMC 2018. Institute of Electrical and Electronics Engineers Inc. pp. 2583-2588. (IEEE International Conference on Systems, Man, and Cybernetics). https://doi.org/10.1109/SMC.2018.00442
Zempeltzi M-M, Kisse M, Brunk MGK, Glemser C, Aksit S, Deane KE, Maurya S, Schneider L, Ohl F, Deliano M, Happel M. 2019. Task rule and choice are reflected by layer-specific processing in rodent auditory cortical microcircuits. bioRxiv. https://doi.org/10.1101/860064
2018
Acosta-Cabronero J, Milovic C, Mattern H, Tejos C, Speck O, Callaghan MF. 2018. A robust multi-scale approach to quantitative susceptibility mapping. NeuroImage. 183:7-24. https://doi.org/10.1016/j.neuroimage.2018.07.065
Annamneedi A, Caliskan G, Müller S, Montag D, Budinger E, Angenstein F, Fejtova A, Tischmeyer W, Gundelfinger ED, Stork O. 2018. Ablation of the presynaptic organizer Bassoon in excitatory neurons retards dentate gyrus maturation and enhances learning performance. Brain Structure and Function. 223(7):3423-3445. https://doi.org/10.1007/s00429-018-1692-3
Bovet-Carmona M, Menigoz A, Pinto S, Tambuyzer T, Krautwald K, Voets T, Aerts JM, Angenstein F, Vennekens R, Balschun D. 2018. Disentangling the role of TRPM4 in hippocampus-dependent plasticity and learning: an electrophysiological, behavioral and FMRI approach. Brain Structure and Function. 223(8):3557-3576. https://doi.org/10.1007/s00429-018-1706-1
Brocka M, Helbing C, Vincenz D, Scherf T, Montag D, Goldschmidt J, Angenstein F, Lippert M. 2018. Contributions of dopaminergic and non-dopaminergic neurons to VTA-stimulation induced neurovascular responses in brain reward circuits. NeuroImage. 177:88-97. https://doi.org/10.1016/j.neuroimage.2018.04.059
Budinger E, Kanold P. 2018. Auditory cortical circuits. Oliver DL, Cant N, Fay RR, Popper AN, editors. In The Mammalian Auditory Pathways: Synaptic Organization and Microcircuits. New York: Springer. pp. 199 - 233. (Springer Handbook of Auditory Research). https://doi.org/10.1007/978-3-319-71798-2_8
Bulitta B, Zuschratter W, Bernal I, Bruder D, Klawonn F, von Bergen M, Garritsen HSP, Jänsch L. 2018. Proteomic definition of human mucosal-associated invariant T cells determines their unique molecular effector phenotype. European Journal of Immunology. 48(8):1336-1349. https://doi.org/10.1002/eji.201747398
Deliano M, Brunk MGK, El-Tabbal M, Zempeltzi MM, Happel MFK, Ohl FW. 2018. Dopaminergic neuromodulation of high gamma stimulus phase-locking in gerbil primary auditory cortex mediated by D1/D5-receptors. European Journal of Neuroscience. https://doi.org/10.1111/ejn.13898
Edelmann B, Gupta N, Schnoeder TM, Oelschlegel AM, Shahzad K, Goldschmidt J, Philipsen L, Weinert S, Ghosh A, Saalfeld FC, Nimmagadda SC, Müller P, Braun-Dullaeus R, Mohr J, Wolleschak D, Kliche S, Amthauer H, Heidel FH, Schraven B, Isermann B, Müller AJ, Fischer T. 2018. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. Journal of Clinical Investigation. 128(10):4359-4371. https://doi.org/10.1172/JCI90312
Friebe B, Richter M, Penzlin S, Stärke C, Kropf S, Lohmann C, Fischbach F, Speck O. 2018. Assessment of Low-Grade Meniscal and Cartilage Damage of the Knee at 7 T: A Comparison to 3 T Imaging with Arthroscopic Correlation. Investigative Radiology. 53(7):390-396. https://doi.org/10.1097/RLI.0000000000000456
Henschke JU, Oelschlegel AM, Angenstein F, Ohl FW, Goldschmidt J, Kanold PO, Budinger E. 2018. Early sensory experience influences the development of multisensory thalamocortical and intracortical connections of primary sensory cortices. Brain Structure and Function. 223(3):1165-1190. https://doi.org/10.1007/s00429-017-1549-1
Henschke JU, Ohl FW, Budinger E. 2018. Crossmodal connections of primary sensory cortices largely vanish during normal aging. Frontiers in Aging Neuroscience. 10(MAR). https://doi.org/10.3389/fnagi.2018.00052
Herrmann T, Liebig T, Mallow J, Bruns C, Stadler J, Mylius J, Brosch M, Svedja JT, Chen Z, Rennings A, Scheich H, Plaumann M, Hauser MJB, Bernarding J, Erni D. 2018. Metamaterial-based transmit and receive system for whole-body magnetic resonance imaging at ultra-high magnetic fields. PLoS ONE. 13(1). https://doi.org/10.1371/journal.pone.0191719
Iliadou VV, Ptok M, Grech H, Pedersen ER, Brechmann A, Deggouj N, Kiese-Himmel C, S´liwin´ska-Kowalska M, Nickisch A, Demanez L, Veuillet E, Thai-Van H, Sirimanna T, Callimachou M, Santarelli R, Kuske S, Barajas de Prat JJ, Hedever M, Konukseven O, Veraguth D, Mattsson TS, Martins JH, Bamiou DE. 2018. European 17 countries consensus endorses more approaches to APD than reported in Wilson 2018. International Journal of Audiology. 57(5):395-396. https://doi.org/10.1080/14992027.2018.1442937
Konugolu Venkata Sekar S, Mosca S, Tannert S, Valentini G, Martelli F, Binzoni T, Prokazov Y, Turbin E, Zuschratter W, Erdmann R, Pifferi A. 2018. Time domain diffuse Raman spectrometer based on a TCSPC camera for the depth analysis of diffusive media. Optics Letters. 43(9):2134-2137. https://doi.org/10.1364/OL.43.002134
Lützkendorf R, Heidemann RM, Feiweier T, Luchtmann M, Baecke S, Kaufmann J, Stadler J, Budinger E, Bernarding J. 2018. Mapping fine-scale anatomy of gray matter, white matter, and trigeminal-root region applying spherical deconvolution to high-resolution 7-T diffusion MRI. Magnetic Resonance Materials in Physics, Biology, and Medicine. 31(6):701-713. https://doi.org/10.1007/s10334-018-0705-9
Mannewitz A, Bock J, Kreitz S, Hess A, Goldschmidt J, Scheich H, Braun K. 2018. Comparing brain activity patterns during spontaneous exploratory and cue-instructed learning using single photon-emission computed tomography (SPECT) imaging of regional cerebral blood flow in freely behaving rats. Brain Structure and Function. 223(4):2025-2038. https://doi.org/10.1007/s00429-017-1605-x
Mattern H, Sciarra A, Godenschweger F, Stucht D, Lüsebrink F, Rose G, Speck O. 2018. Prospective motion correction enables highest resolution time-of-flight angiography at 7T. Magnetic Resonance in Medicine. 80(1):248-258. https://doi.org/10.1002/mrm.27033
Mikhaylova M, Bär J, van Bommel B, Schätzle P, YuanXiang PA, Raman R, Hradsky J, Konietzny A, Loktionov EY, Reddy PP, Lopez-Rojas J, Spilker C, Kobler O, Raza SA, Stork O, Hoogenraad CC, Kreutz MR. 2018. Caldendrin Directly Couples Postsynaptic Calcium Signals to Actin Remodeling in Dendritic Spines. Neuron. 97(5):1110-1125.e14. https://doi.org/10.1016/j.neuron.2018.01.046
Peitek N, Siegmund J, Apel S, Kastner C, Parnin C, Bethmann A, Leich T, Saake G, Brechmann A. 2018. A Look into Programmers Heads. IEEE Transactions on Software Engineering. https://doi.org/10.1109/TSE.2018.2863303
Peitek N, Siegmund J, Parnin C, Apel S, Hofmeister J, Brechmann A. 2018. Simultaneous Measurement of Program Comprehension with fMRI and Eye Tracking: A Case Study. In Proceedings of the 12th ACM/IEEE International Symposium on Empirical Software Engineering and Measurement, ESEM 2018. IEEE Computer Society. (IEEE International Symposium on Empirical Software Engineering and Measurement). https://doi.org/10.1145/3239235.3240495
Peitek N. 2018. A neuro-cognitive perspective of program comprehension. In Proceedings - International Conference on Software Engineering. IEEE Computer Society. pp. 496-499. (Proceedings - International Conference on Software Engineering). https://doi.org/10.1145/3183440.3183442
Saumweber T, Rohwedder A, Schleyer M, Eichler K, Chen Y-C, Aso Y, Cardona A, Eschbach C, Kobler O, Voigt A, Durairaja A, Mancini N, Zlatic M, Truman JW, Thum A, Gerber B. 2018. Functional architecture of reward learning in mushroom body extrinsic neurons of larval Drosophila. Nature Communications. 9(1). https://doi.org/10.1038/s41467-018-03130-1
Sekar SKV, Mosca S, Valentini G, Zuschratter W, Erdmann R, Pifferi A. 2018. Compact time domain diffuse raman instrumentation based on a TCSPC camera for depth probing of diffusive media. In Optical Tomography and Spectroscopy, OTS 2018. OSA - The Optical Society. https://doi.org/10.1364/OTS.2018.OTu4D.2
Selezneva E, Gorkin A, Budinger E, Brosch M. 2018. Neuronal correlates of auditory streaming in the auditory cortex of behaving monkeys. European Journal of Neuroscience. 48(10):3234-3245. https://doi.org/10.1111/ejn.14098
Siegert I, Lotz AF, Egorow O, Wolff S. 2018. Utilizing Psychoacoustic Modeling to Improve Speech-Based Emotion Recognition. Potapova R, Jokisch O, Karpov A, editors. In Speech and Computer - 20th International Conference, SPECOM 2018, Proceedings. Springer Verlag. pp. 625-635. (Lecture Notes in Computer Science). https://doi.org/10.1007/978-3-319-99579-3_64
2017
Angenstein N, Brechmann A. 2017. Effect of sequential comparison on active processing of sound duration. Human Brain Mapping. 38(9):4459-4469. https://doi.org/10.1002/hbm.23673
Angus DJ, Latham AJ, Harmon-Jones E, Deliano M, Balleine B, Braddon-Mitchell D. 2017. Electrocortical components of anticipation and consumption in a monetary incentive delay task. Psychophysiology. 54(11):1686-1705. https://doi.org/10.1111/psyp.12913
Bhattacharya S, Herrera-Molina R, Sabanov V, Ahmed T, Iscru E, Stöber F, Richter K, Fischer KD, Angenstein F, Goldschmidt J, Beesley PW, Balschun D, Smalla KH, Gundelfinger ED, Montag D. 2017. Genetically induced retrograde amnesia of associative memories after neuroplastin ablation. Biological Psychiatry. 81(2):124-135. https://doi.org/10.1016/j.biopsych.2016.03.2107
Helbing C, Tischmeyer W, Angenstein F. 2017. Late effect of dopamine D1/5 receptor activation on stimulus-induced BOLD responses in the hippocampus and its target regions depends on the history of previous stimulations. NeuroImage. 152:119-129. https://doi.org/10.1016/j.neuroimage.2017.02.077
Herrera-Molina R, Mlinac-Jerkovic K, Ilic K, Stöber F, Vemula SK, Sandoval M, Milosevic NJ, Simic G, Smalla KH, Goldschmidt J, Bognar SK, Montag D. 2017. Neuroplastin deletion in glutamatergic neurons impairs selective brain functions and calcium regulation: Implication for cognitive deterioration. Scientific Reports. 7(1). https://doi.org/10.1038/s41598-017-07839-9
Iliadou V, Ptok M, Grech H, Pedersen ER, Brechmann A, Deggouj N, Kiese-Himmel C, Sliwinska-Kowalska M, Nickisch A, Demanez L, Veuillet E, Thai-Van H, Sirimanna T, Callimachou M, Santarelli R, Kuske S, Barajas J, Hedjever M, Konukseven O, Veraguth D, Mattsson TS, Martins JH, Bamiou DE. 2017. A European perspective on auditory processing disorder-current knowledge and future research focus. Frontiers in Neurology. 8(NOV). https://doi.org/10.3389/fneur.2017.00622
In MH, Posnansky O, Speck O. 2017. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. NeuroImage. 148:20-30. https://doi.org/10.1016/j.neuroimage.2017.01.008
Junghans C, Vukojević V, Tavraz NN, Maksimov EG, Zuschratter W, Schmitt FJ, Friedrich T. 2017. Disruption of Ankyrin B and Caveolin-1 Interaction Sites Alters Na+,K+-ATPase Membrane Diffusion. Biophysical Journal. 113(10):2249-2260. https://doi.org/10.1016/j.bpj.2017.08.053
Klasvogt S, Zuschratter W, Schmidt A, Kröber A, Vorwerk S, Wolter R, Isermann B, Wimmers K, Rothkötter HJ, Nossol C. 2017. Air-liquid interface enhances oxidative phosphorylation in intestinal epithelial cell line IPEC-J2. Cell Death Discovery. 3:17001. https://doi.org/10.1038/cddiscovery.2017.1
Korthals M, Langnaese K, Smalla KH, Kähne T, Herrera-Molina R, Handschuh J, Lehmann AC, Mamula D, Naumann M, Seidenbecher C, Zuschratter W, Tedford K, Gundelfinger ED, Montag D, Fischer KD, Thomas U. 2017. A complex of Neuroplastin and Plasma Membrane Ca2+ ATPase controls T cell activation. Scientific Reports. 7(1). https://doi.org/10.1038/s41598-017-08519-4
Low T, Bubalo N, Gossen T, Kotzyba M, Brechmann A, Huckauf A, Nürnberger A. 2017. Towards identifying user intentions in exploratory search using gaze and pupil tracking. In CHIIR 2017 - Proceedings of the 2017 Conference Human Information Interaction and Retrieval . Association for Computing Machinery, Inc. pp. 273-276. https://doi.org/10.1145/3020165.3022131
Maldonado H, Calderon C, Burgos-Bravo F, Kobler O, Zuschratter W, Ramirez O, Härtel S, Schneider P, Quest AFG, Herrera-Molina R, Leyton L. 2017. Astrocyte-to-neuron communication through integrin-engaged Thy-1/CBP/Csk/Src complex triggers neurite retraction via the RhoA/ROCK pathway. Biochimica et Biophysica Acta - Molecular Cell Research. 1864(2):243-254. https://doi.org/10.1016/j.bbamcr.2016.11.006
Parnin C, Siegmund J, Peitek N. 2017. On the Nature of Programmer Expertise. In 28th Annual Workshop of the Psychology of Programming Interest Group (PPIG).
Philipsen L, Reddycherla AV, Hartig R, Gumz J, Kästle M, Kritikos A, Poltorak MP, Prokazov Y, Turbin E, Weber A, Zuschratter W, Schraven B, Simeoni L, Müller AJ. 2017. De novo phosphorylation and conformational opening of the tyrosine kinase Lck act in concert to initiate T cell receptor signaling. Science Signaling. 10(462). https://doi.org/10.1126/scisignal.aaf4736
Pöttker B, Stöber F, Hummel R, Angenstein F, Radyushkin K, Goldschmidt J, Schäfer MKE. 2017. Traumatic brain injury causes long-term behavioral changes related to region-specific increases of cerebral blood flow. Brain Structure and Function. 222(9):4005-4021. https://doi.org/10.1007/s00429-017-1452-9
Prezenski S, Brechmann A, Wolff S, Russwinkel N. 2017. A cognitive modeling approach to strategy formation in dynamic decision making. Frontiers in Psychology. 8(AUG). https://doi.org/10.3389/fpsyg.2017.01335
Riemann S, Helbing C, Angenstein F. 2017. From unspecific to adjusted, how the BOLD response in the rat hippocampus develops during consecutive stimulations. Journal of Cerebral Blood Flow and Metabolism. 37(2):590-604. https://doi.org/10.1177/0271678X16634715
Scherf T, Angenstein F. 2017. Hippocampal CA3 activation alleviates fMRI-BOLD responses in the rat prefrontal cortex induced by electrical VTA stimulation. PLoS ONE. 12(2). https://doi.org/10.1371/journal.pone.0172926
Schulz J, Rohracker M, Stiebler M, Goldschmidt J, Stöber F, Noriega M, Pethe A, Lukas M, Osterkamp F, Reineke U, Höhne A, Smerling C, Amthauer H. 2017. Proof of therapeutic efficacy of a 177Lu-labeled neurotensin receptor 1 antagonist in a colon carcinoma xenograft model. Journal of Nuclear Medicine. 58(6):936-941. https://doi.org/10.2967/jnumed.116.185140
Siegmund J, Peitek N, Parnin C, Apel S, Hofmeister J, Kästner C, Begel A, Bethmann A, Brechmann A. 2017. Measuring Neural Efficiency of Program Comprehension. In Proceedings of the 2017 11th Joint Meeting on Foundations of Software Engineering. AMC Press. pp. 140-150.
Töpperwien M, Krenkel M, Vincenz D, Stöber F, Oelschlegel AM, Goldschmidt J, Salditt T. 2017. Three-dimensional mouse brain cytoarchitecture revealed by laboratory-based x-ray phase-contrast tomography. Scientific Reports. 7. https://doi.org/10.1038/srep42847
Vincenz D, Wernecke KEA, Fendt M, Goldschmidt J. 2017. Habenula and interpeduncular nucleus differentially modulate predator odor-induced innate fear behavior in rats. Behavioural Brain Research. 332:164-171. https://doi.org/10.1016/j.bbr.2017.05.053
Wagner M, Baer C, Zuschratter W, Riek-Burchardt M, Deffge C, Weinert S, Lee JC, Braun-Dullaeus RC, Herold J. 2017. Intravital Microscopy of Monocyte Homing and Tumor-Related Angiogenesis in a Murine Model of Peripheral Arterial Disease. Journal of Visualized Experiments. (126). https://doi.org/10.3791/56290
Weber A, Prokazov Y, Zuschratter W, Hauser MJB. 2017. From synchronised to desynchronised glycolytic oscillations in individual yeast cells. Müller SC, Plath PJ, Radons G, Fuchs A, editors. In Complexity and Synergetics. Springer. pp. 239-254. (Complexity and Synergetics). https://doi.org/10.1007/978-3-319-64334-2_19
Yakupov R, Lei J, Hoffmann MB, Speck O. 2017. False fMRI activation after motion correction. Human Brain Mapping. 38(9):4497-4510. https://doi.org/10.1002/hbm.23677
Yarach U, In MH, Chatnuntawech I, Bilgic B, Godenschweger F, Mattern H, Sciarra A, Speck O. 2017. Model-based iterative reconstruction for single-shot EPI at 7T. Magnetic Resonance in Medicine. 78(6):2250-2264. https://doi.org/10.1002/mrm.26633
Zhao B, Meka DP, Scharrenberg R, König T, Schwanke B, Kobler O, Windhorst S, Kreutz MR, Mikhaylova M, Calderon De Anda F. 2017. Microtubules Modulate F-actin Dynamics during Neuronal Polarization. Scientific Reports. 7(1). https://doi.org/10.1038/s41598-017-09832-8
2016
Angenstein N, Stadler J, Brechmann A. 2016. Auditory intensity processing: Effect of MRI background noise. Hearing Research. 333:87-92. https://doi.org/10.1016/j.heares.2016.01.007
Deike S, Deliano M, Brechmann A. 2016. Probing neural mechanisms underlying auditory stream segregation in humans by transcranial direct current stimulation (tDCS). Neuropsychologia. 91:262-267. https://doi.org/10.1016/j.neuropsychologia.2016.08.017
Deliano M, Tabelow K, König R, Polzehl J. 2016. Improving accuracy and temporal resolution of learning curve estimation for within- and across-session analysis. PLoS ONE. 11(6):Article e0157355. https://doi.org/10.1371/journal.pone.0157355
Ferrando-May E, Hartmann H, Reymann J, Ansari N, Utz N, Fried HU, Kukat C, Peychl J, Liebig C, Terjung S, Laketa V, Sporbert A, Weidtkamp-Peters S, Schauss A, Zuschratter W, Avilov S. 2016. Advanced light microscopy core facilities: Balancing service, science and career. Microscopy Research and Technique. 79(6):463-479. https://doi.org/10.1002/jemt.22648
Godenschweger F, Kägebein U, Stucht D, Yarach U, Sciarra A, Yakupov R, Lüsebrink F, Schulze P, Speck O. 2016. Motion correction in MRI of the brain. Physics in Medicine and Biology. 61(5):R32-R56. https://doi.org/10.1088/0031-9155/61/5/R32
Hanke M, Adelhöfer N, Kottke D, Iacovella V, Sengupta A, Kaule FR, Nigbur R, Waite AQ, Baumgartner F, Stadler J. 2016. A studyforrest extension, simultaneous fMRI and eye gaze recordings during prolonged natural stimulation. Scientific data. 3:Article 160092. https://doi.org/10.1038/sdata.2016.92
In MH, Posnansky O, Speck O. 2016. PSF mapping-based correction of eddy-current-induced distortions in diffusion-weighted echo-planar imaging. Magnetic Resonance in Medicine. 75(5):2055-2063. https://doi.org/10.1002/mrm.25746
Jarvers C, Brosch T, Brechmann A, Woldeit ML, Schulz AL, Ohl FW, Lommerzheim M, Neumann H. 2016. Reversal learning in humans and gerbils: Dynamic control network facilitates learning. Frontiers in Neuroscience. 10(NOV):Article 535. https://doi.org/10.3389/fnins.2016.00535
Kohrs C, Angenstein N, Brechmann A. 2016. Delays in human-computer interaction and their effects on brain activity. PLoS ONE. 11(1):Article e0146250. https://doi.org/10.1371/journal.pone.0146250
Medunjanin S, Schleithoff L, Fiegehenn C, Weinert S, Zuschratter W, Braun-Dullaeus RC. 2016. GSK-3β controls NF-kappaB activity via IKKγ/NEMO. Scientific Reports. 6:Article 38553. https://doi.org/10.1038/srep38553
Mikhaylova M, Bera S, Kobler O, Frischknecht R, Kreutz MR. 2016. A Dendritic Golgi Satellite between ERGIC and Retromer. Cell Reports. 14(2):189-199. https://doi.org/10.1016/j.celrep.2015.12.024
Radtke-Schuller S, Schuller G, Angenstein F, Grosser OS, Goldschmidt J, Budinger E. 2016. Brain atlas of the Mongolian gerbil (Meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Structure and Function. 221 Suppl 1(Suppl. 1):1-272. https://doi.org/10.1007/s00429-016-1259-0
Rösner D, Hazer-Rau D, Kohrs C, Bauer T, Günther S, Hoffmann H, Zhang L, Brechmann A. 2016. Is there a biological basis for success in human companion interaction? Results from a transsituational study. in Human-Computer Interaction. Theory, Design, Development and Practice. Springer Verlag. S. 77-88. (Lecture Notes in Computer Science). https://doi.org/10.1007/978-3-319-39510-4_8
Schulz J, Rohracker M, Stiebler M, Goldschmidt J, Grosser OS, Osterkamp F, Pethe A, Reineke U, Smerling C, Amthauer H. 2016. Comparative evaluation of the biodistribution profiles of a series of nonpeptidic neurotensin receptor-1 antagonists reveals a promising candidate for theranostic applications. Journal of Nuclear Medicine. 57(7):1120-1123. https://doi.org/10.2967/jnumed.115.170530
Sengupta A, Kaule FR, Guntupalli JS, Hoffmann MB, Häusler C, Stadler J, Hanke M. 2016. A studyforrest extension, retinotopic mapping and localization of higher visual areas. Scientific data. 3:Article 160093. https://doi.org/10.1038/sdata.2016.93
Spilker C, Nullmeier S, Grochowska KM, Schumacher A, Butnaru I, Macharadze T, Gomes GM, YuanXiang P, Bayraktar G, Rodenstein C, Geiseler C, Kolodziej A, Lopez-Rojas J, Montag D, Angenstein F, Bär J, D’Hanis W, Roskoden T, Mikhaylova M, Budinger E, Ohl FW, Stork O, Zenclussen AC, Karpova A, Schwegler H, Kreutz MR. 2016. A Jacob/Nsmf Gene Knockout Results in Hippocampal Dysplasia and Impaired BDNF Signaling in Dendritogenesis. PLoS Genetics. 12(3):Article e1005907. https://doi.org/10.1371/journal.pgen.1005907
Tran VL, Génot V, Audibert J-F, Prokazov Y, Turbin E, Zuschratter W, Kim H-J, Jung C, Park SY, Pansu RB. 2016. Nucleation and growth during a fluorogenic precipitation in a micro-flow mapped by fluorescence lifetime microscopy. New Journal of Chemistry. 40(5):4601-4605. https://doi.org/10.1039/c5nj03400k
Vogel H, Wolf S, Rabasa C, Rodriguez-Pacheco F, Babaei CS, Stöber F, Goldschmidt J, DiMarchi RD, Finan B, Tschöp MH, Dickson SL, Schürmann A, Skibicka KP. 2016. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology. 110:396-406. https://doi.org/10.1016/j.neuropharm.2016.07.039
Yarach U, Luengviriya C, Stucht D, Godenschweger F, Schulze P, Speck O. 2016. Correction of B0-induced geometric distortion variations in prospective motion correction for 7T MRI. Magnetic Resonance Materials in Physics, Biology, and Medicine. 29(3):319-332. https://doi.org/10.1007/s10334-015-0515-2
2015
Angenstein N, Brechmann A. 2015. Auditory intensity processing: Categorization versus comparison. NeuroImage. 119:362-370. https://doi.org/10.1016/j.neuroimage.2015.06.074
Baecke S, Lützkendorf R, Mallow J, Luchtmann M, Tempelmann C, Stadler J, Bernarding J. 2015. A proof-of-principle study of multi-site real-time functional imaging at 3T and 7T: Implementation and validation. Scientific Reports. 5:8413. https://doi.org/10.1038/srep08413
Budinger E, Brechmann A, Brosch M, Heil P, König R, Ohl FW, Scheich H. 2015. Auditory cortex 2014 - towards a synthesis of human and animal research. European Journal of Neuroscience. 41(5):515-517. https://doi.org/10.1111/ejn.12832
Deike S, Heil P, Böckmann-Barthel M, Brechmann A. 2015. Decision making and ambiguity in auditory stream segregation. Frontiers in Neuroscience. 9(JUL):Article 266. https://doi.org/10.3389/fnins.2015.00266
Erdmann I, Marter K, Kobler O, Niehues S, Abele J, Müller A, Bussmann J, Storkebaum E, Ziv T, Thomas U, Dieterich DC. 2015. Cell-selective labelling of proteomes in Drosophila melanogaster. Nature Communications. 6:Article 7521. https://doi.org/10.1038/ncomms8521
Fidzinski P, Korotkova T, Heidenreich M, Maier N, Schuetze S, Kobler O, Zuschratter W, Schmitz D, Ponomarenko A, Jentsch TJ. 2015. KCNQ5 K+ channels control hippocampal synaptic inhibition and fast network oscillations. Nature Communications. 6:Article 7254. https://doi.org/10.1038/ncomms7254
Hanke M, Dinga R, Häusler C, Guntupalli JS, Casey M, Kaule FR, Stadler J. 2015. High-resolution 7-Tesla fMRI data on the perception of musical genres – an extension to the studyforrest dataset. F1000Research. https://doi.org/10.12688/f1000research.6679.1
Happel MFK, Deliano M, Ohl FW. 2015. Combined shuttle-box training with electrophysiological cortex recording and stimulation as a tool to study perception and learning. Journal of Visualized Experiments. 2015(105):Article e53002. https://doi.org/10.3791/53002
Henschke JU, Noesselt T, Scheich H, Budinger E. 2015. Possible anatomical pathways for short-latency multisensory integration processes in primary sensory cortices. Brain Structure and Function. 220(2):955-977. https://doi.org/10.1007/s00429-013-0694-4
Herrmann T, Mallow J, Plaumann M, Luchtmann M, Stadler J, Mylius J, Brosch M, Bernarding J. 2015. The travelling-wave Primate System: A new solution for magnetic resonance imaging of macaque monkeys at 7 tesla ultra-high field. PLoS ONE. 10(6):Article e0129371. https://doi.org/10.1371/journal.pone.0129371
In MH, Posnansky O, Beall EB, Lowe MJ, Speck O. 2015. Distortion correction in EPI using an extended PSF method with a reversed phase gradient approach. PLoS ONE. 10(2):Article e0116320. https://doi.org/10.1371/journal.pone.0116320
Janitzky K, Lippert MT, Engelhorn A, Tegtmeier J, Goldschmidt J, Heinze HJ, Ohl FW. 2015. Optogenetic silencing of locus coeruleus activity in mice impairs cognitive flexibility in an attentional set-shifting task. Frontiers in Behavioral Neuroscience. 9(NOVEMBER):Article 286. https://doi.org/10.3389/fnbeh.2015.00286
Krippl M, Karim AA, Brechmann A. 2015. Neuronal correlates of voluntary facial movements. Frontiers in Human Neuroscience. 9(OCTOBER):Article 598. https://doi.org/10.3389/fnhum.2015.00598
Mallow J, Bernarding J, Luchtmann M, Bethmann A, Brechmann A. 2015. Superior memorizers employ different neural networks for encoding and recall. Frontiers in Systems Neuroscience. 9(September):Article A128. https://doi.org/10.3389/fnsys.2015.00128
Medunjanin S, Daniel JM, Weinert S, Dutzmann J, Burgbacher F, Brecht S, Bruemmer D, Kähne T, Naumann M, Sedding DG, Zuschratter W, Braun-Dullaeus RC. 2015. DNA-dependent protein kinase (DNA-PK) permits vascular smooth muscle cell proliferation through phosphorylation of the orphan nuclear receptor NOR1. Cardiovascular Research. 106(3):488-497. https://doi.org/10.1093/cvr/cvv126
Nossol C, Barta-Böszörményi A, Kahlert S, Zuschratter W, Faber-Zuschratter H, Reinhardt N, Ponsuksili S, Wimmers K, Diesing A, Rothkötter HJ. 2015. Comparing two intestinal porcine epithelial cell lines (IPECs): Morphological differentiation, function and metabolism. PLoS ONE. 10(7):Article e132323. https://doi.org/10.1371/journal.pone.0132323
Rothe T, Deliano M, Wójtowicz AM, Dvorzhak A, Harnack D, Paul S, Vagner T, Melnick I, Stark H, Grantyn R. 2015. Pathological gamma oscillations, impaired dopamine release, synapse loss and reduced dynamic range of unitary glutamatergic synaptic transmission in the striatum of hypokinetic Q175 Huntington mice. Neuroscience. 311:519-538. https://doi.org/10.1016/j.neuroscience.2015.10.039
Scherf T, Angenstein F. 2015. Postsynaptic and spiking activity of pyramidal cells, the principal neurons in the rat hippocampal CA1 region, does not control the resultant BOLD response: A combined electrophysiologic and fMRI approach. Journal of Cerebral Blood Flow and Metabolism. 35(4):565-575. https://doi.org/10.1038/jcbfm.2014.252
Specht K, Baumgartner FJ, Stadler J, Hugdahl K, Pollmann S. 2015. Functional asymmetry and effective connectivity of the auditory system during speech perception is modulated by the place of articulation of the consonant – A 7 T fMRI study. in LATERALIZATION AND COGNITIVE SYSTEMS. Frontiers Media S. A. S. 180-189. https://doi.org/10.3389/978-2-88919-411-7
Stucht D, Danishad KA, Schulze P, Godenschweger F, Zaitsev M, Speck O. 2015. Highest resolution in vivo human brain MRI using prospective motion correction. PLoS ONE. 10(7):e0133921. https://doi.org/10.1371/journal.pone.0133921
Wanger T, Wetzel W, Scheich H, Ohl FW, Goldschmidt J. 2015. Spatial patterns of neuronal activity in rat cerebral cortex during non-rapid eye movement sleep. Brain Structure and Function. 220(6):3469-3484. https://doi.org/10.1007/s00429-014-0867-9
Wernecke KEA, Vincenz D, Storsberg S, D'Hanis W, Goldschmidt J, Fendt M. 2015. Fox urine exposure induces avoidance behavior in rats and activates the amygdalar olfactory cortex. Behavioural Brain Research. 279:76-81. https://doi.org/10.1016/j.bbr.2014.11.020
Wolff S, Brechmann A. 2015. Carrot and stick 2.0: The benefits of natural and motivational prosody in computer-assisted learning. Computers in Human Behavior. 43:76-84. https://doi.org/10.1016/j.chb.2014.10.015
Yarach U, Luengviriya C, Danishad A, Stucht D, Godenschweger F, Schulze P, Speck O. 2015. Correction of gradient nonlinearity artifacts in prospective motion correction for 7T MRI. Magnetic Resonance in Medicine. 73(4):1562-1569. https://doi.org/10.1002/mrm.25283
2014
Angenstein F. 2014. The actual intrinsic excitability of granular cells determines the ruling neurovascular coupling mechanism in the rat dentate gyrus. Journal of Neuroscience. 34(25):8529-8545. https://doi.org/10.1523/JNEUROSCI.0472-14.2014
Bethmann A, Brechmann A. 2014. On the definition and interpretation of voice selective activation in the temporal cortex. Frontiers in Human Neuroscience. 8(JULY):Article 499. https://doi.org/10.3389/fnhum.2014.00499
Böckmann-Barthel M, Deike S, Brechmann A, Ziese M, Verhey JL. 2014. Time course of auditory streaming: Do CI users differ from normal-hearing listeners?. Frontiers in Psychology. 5(JUL):Article 775. https://doi.org/10.3389/fpsyg.2014.00775
Braun J, Guo J, Lützkendorf R, Stadler J, Papazoglou S, Hirsch S, Sack I, Bernarding J. 2014. High-resolution mechanical imaging of the human brain by three-dimensional multifrequency magnetic resonance elastography at 7T. NeuroImage. 90:308-314. https://doi.org/10.1016/j.neuroimage.2013.12.032
Budinger E, Hrsg. 2014. Towards a Synthesis of Human and Animal Research: Proceedings of the 5th International Conference on Auditory Cortex. Magdeburg, Germany: Docupoint Verlag. 158 S.
Dolležal LV, Brechmann A, Klump GM, Deike S. 2014. Evaluating auditory stream segregation of SAM tone sequences by subjective and objective psychoacoustical tasks, and brain activity. Frontiers in Neuroscience. 8(8 JUN):Article 119. https://doi.org/10.3389/fnins.2014.00119
Hanke M, Baumgartner FJ, Ibe P, Kaule FR, Pollmann S, Speck O, Zinke W, Stadler J. 2014. A high-resolution 7-Tesla fMRI dataset from complex natural stimulation with an audio movie. Scientific data. 1:Article 140003. https://doi.org/10.1038/sdata.2014.3
Happel MFK, Deliano M, Handschuh J, Ohl FW. 2014. Dopamine-modulated recurrent corticoefferent feedback in primary sensory cortex promotes detection of behaviorally relevant stimuli. Journal of Neuroscience. 34(4):1234-1247. https://doi.org/10.1523/JNEUROSCI.1990-13.2014
Happel MFK, Niekisch H, Castiblanco Rivera LL, Ohl FW, Deliano M, Frischknecht R. 2014. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 111(7):2800-2805. https://doi.org/10.1073/pnas.1310272111
Hartig R, Prokazov Y, Turbin E, Zuschratter W. 2014. Wide-Field fluorescence lifetime imaging with multi-anode detectors. in Fluorescence Spectroscopy and Microscopy: Methods and Protocols. Humana Press Inc. S. 457-480. (Methods in Molecular Biology). https://doi.org/10.1007/978-1-62703-649-8_20
Hunger M, Budinger E, Zhong K, Angenstein F. 2014. Visualization of acute focal lesions in rats with experimental autoimmune encephalomyelitis by magnetic nanoparticles, comparing different MRI sequences including phase imaging. Journal of Magnetic Resonance Imaging. 39(5):1126-1135. https://doi.org/10.1002/jmri.24280
Kanowski M, Voges J, Buentjen L, Stadler J, Heinze HJ, Tempelmann C. 2014. Direct visualization of anatomic subfields within the superior aspect of the human lateral thalamus by MRI at 7T. American Journal of Neuroradiology. 35(9):1721-1727. https://doi.org/10.3174/ajnr.A3951
Kaule FR, Wolynski B, Gottlob I, Stadler J, Speck O, Kanowski M, Meltendorf S, Behrens-Baumann W, Hoffmann MB. 2014. Impact of chiasma opticum malformations on the organization of the human ventral visual cortex. Human Brain Mapping. 35(10):5093-5105. https://doi.org/10.1002/hbm.22534
Koch N, Kobler O, Thomas U, Qualmann B, Kessels MM. 2014. Terminal axonal arborization and synaptic bouton formation critically rely on Abp1 and the Arp2/3 complex. PLoS ONE. 9(5):Article e97692. https://doi.org/10.1371/journal.pone.0097692
Kohrs C, Hrabal D, Angenstein N, Brechmann A. 2014. Delayed system response times affect immediate physiology and the dynamics of subsequent button press behavior. Psychophysiology. 51(11):1178-1184. https://doi.org/10.1111/psyp.12253
Kolodziej A, Lippert M, Angenstein F, Neubert J, Pethe A, Grosser OS, Amthauer H, Schroeder UH, Reymann KG, Scheich H, Ohl FW, Goldschmidt J. 2014. SPECT-imaging of activity-dependent changes in regional cerebral blood flow induced by electrical and optogenetic self-stimulation in mice. NeuroImage. 103:171-180. https://doi.org/10.1016/j.neuroimage.2014.09.023
Lison H, Happel MFK, Schneider F, Baldauf K, Kerbstat S, Seelbinder B, Schneeberg J, Zappe M, Goldschmidt J, Budinger E, Schröder UH, Ohl FW, Schilling S, Demuth HU, Scheich H, Reymann KG, Rönicke R. 2014. Disrupted cross-laminar cortical processing in β amyloid pathology precedes cell death. Neurobiology of Disease. 63:62-73. https://doi.org/10.1016/j.nbd.2013.11.014
Mikhaylova M, Karpova A, Bär J, Bethge P, YuanXiang P, Chen Y, Zuschratter W, Behnisch T, Kreutz MR. 2014. Cellular distribution of the NMDA-receptor activated synapto-nuclear messenger Jacob in the rat brain. Brain Structure and Function. 219(3):843-860. https://doi.org/10.1007/s00429-013-0539-1
Prokazov Y, Turbin E, Weber A, Hartig R, Zuschratter W. 2014. Position sensitive detector for fluorescence lifetime imaging. Journal of Instrumentation. 9(12):Article C12015. https://doi.org/10.1088/1748-0221/9/12/C12015
Rauschenberg J, Nagel AM, Ladd SC, Theysohn JM, Ladd ME, Möller HE, Trampel R, Turner R, Pohmann R, Scheffler K, Brechmann A, Stadler J, Felder J, Shah NJ, Semmler W. 2014. Multicenter study of subjective acceptance during magnetic resonance imaging at 7 and 9.4 T. Investigative Radiology. 49(5):249-259. https://doi.org/10.1097/RLI.0000000000000035
Rehberg K, Kliche S, Madencioglu DA, Thiere M, Müller B, Manuel Meineke B, Freund C, Budinger E, Stork O. 2014. The serine/threonine kinase Ndr2 controls integrin trafficking and integrin-dependent neurite growth. Journal of Neuroscience. 34(15):5342-5354. https://doi.org/10.1523/JNEUROSCI.2728-13.2014
Saldeitis K, Happel MFK, Ohl FW, Scheich H, Budinger E. 2014. Anatomy of the auditory thalamocortical system in the mongolian gerbil: Nuclear origins and cortical field-, layer-, and frequency-specificities. Journal of Comparative Neurology. 522(10):2397-2430. https://doi.org/10.1002/cne.23540
Seidensticker M, Ulrich G, Muehlberg FL, Pethe A, Grosser OS, Steffen IG, Stiebler M, Goldschmidt J, Smalla KH, Seidensticker R, Ricke J, Amthauer H, Mohnike K. 2014. Tumor cell uptake of 99mTc-Labeled 1-Thio-β-d-glucose and 5-thio-d-glucose in comparison with 2-deoxy-2-[18 f]fluoro-d-glucose in vitro: Kinetics, dependencies, blockage and cell compartment of accumulation. Molecular Imaging and Biology. 16(2):189-198. https://doi.org/10.1007/s11307-013-0690-3
Siegmund J, Kästner C, Apel S, Parnin C, Bethmann A, Leich T, Saake G, Brechmann A. 2014. Understanding understanding source code with functional magnetic resonance imaging. in ICSE 2014 Proceedings of the 36th International Conference on Software Engineering . 1 Aufl. ACM. S. 378-389. https://doi.org/10.1145/2568225.2568252
Specht K, Baumgartner F, Stadler J, Hugdahl K, Pollmann S. 2014. Functional asymmetry and effective connectivity of the auditory system during speech perception is modulated by the place of articulation of the consonant: A 7T fMRI study. Frontiers in Psychology. 5:549. https://doi.org/10.3389/fpsyg.2014.00549
Stöber F, Baldauf K, Ziabreva I, Harhausen D, Zille M, Neubert J, Reymann KG, Scheich H, Dirnagl U, Schröder UH, Wunder A, Goldschmidt J. 2014. Single-cell resolution mapping of neuronal damage in acute focal cerebral ischemia using thallium autometallography. Journal of Cerebral Blood Flow and Metabolism. 34(1):144-152. https://doi.org/10.1038/jcbfm.2013.177
Wasserthal C, Brechmann A, Stadler J, Fischl B, Engel K. 2014. Localizing the human primary auditory cortex in vivo using structural MRI. NeuroImage. 93 Pt 2(P1):237-251. https://doi.org/10.1016/j.neuroimage.2013.07.046
2013
Angenstein F, Krautwald K, Wetzel W, Scheich H. 2013. Perforant pathway stimulation as a conditioned stimulus for active avoidance learning triggers BOLD responses in various target regions of the hippocampus: A combined fMRI and electrophysiological study. NeuroImage. 75:213-227. https://doi.org/10.1016/j.neuroimage.2013.03.007
Angenstein N, Brechmann A. 2013. Division of labor between left and right human auditory cortices during the processing of intensity and duration. NeuroImage. 83:1-11. https://doi.org/10.1016/j.neuroimage.2013.06.071
Angenstein N, Brechmann A. 2013. Left auditory cortex is involved in pairwise comparisons of the direction of frequency modulated tones. Frontiers in Neuroscience. 7(7 JUL):Article Article 115. https://doi.org/10.3389/fnins.2013.00115
Bonath B, Tyll S, Budinger E, Krauel K, Hopf JM, Noesselt T. 2013. Task-demands and audio-visual stimulus configurations modulate neural activity in the human thalamus. NeuroImage. 66:110-118. https://doi.org/10.1016/j.neuroimage.2012.10.018
Brosch M, Budinger E, Scheich H. 2013. Different Synchronization Rules in Primary and Nonprimary Auditory Cortex of Monkeys. Journal of Cognitive Neuroscience. 25(9):1517-1526. https://doi.org/10.1162/jocn_a_00413
Budinger E, Brosch M, Scheich H, Mylius J. 2013. The subcortical auditory structures in the Mongolian gerbil: II. Frequency-related topography of the connections with cortical field AI. Journal of Comparative Neurology. 521(12):2772-2797. https://doi.org/10.1002/cne.23314
Deliano M. 2013. On the intimate relationship between Man and Machine. Epistemology & Philosophy of Science. Alfa-M. S. 141-163.
Diesing AK, Nossol C, Faber-Zuschratter H, Zuschratter W, Renner L, Sokolova O, Naumann M, Rothkötter HJ. 2013. Rapid interaction of helicobacter pylori with microvilli of the polar human gastric epithelial cell line NCI-N87. Anatomical Record. 296(12):1800-1805. https://doi.org/10.1002/ar.22818
Helbing C, Werner G, Angenstein F. 2013. Variations in the temporal pattern of perforant pathway stimulation control the activity in the mesolimbic pathway. NeuroImage. 64(1):43-60. https://doi.org/10.1016/j.neuroimage.2012.09.001
Hrabal D, Kohrs C, Brechmann A, Tan JW, Rukavina S, Traue HC. 2013. Physiological effects of delayed system response time on skin conductance. in Multimodal Pattern Recognition of Social Signals in Human-Computer-Interaction - First IAPR TC3 Workshop, MPRSS 2012, Revised Selected Papers. Springer Nature. S. 52-62. (Lecture Notes in Computer Science). https://doi.org/10.1007/978-3-642-37081-6_7
Hradsky J, Mikhaylova M, Karpova A, Kreutz MR, Zuschratter W. 2013. Super-resolution microscopy of the neuronal calcium-binding proteins calneuron-1 and caldendrin. in Methods in Molecular Biology. S. 147-169. (Methods in molecular biology (Clifton, N.J.)). https://doi.org/10.1007/978-1-62703-230-8_10
Karpova A, Mikhaylova M, Bera S, Bär J, Reddy PP, Behnisch T, Rankovic V, Spilker C, Bethge P, Sahin J, Kaushik R, Zuschratter W, Kähne T, Naumann M, Gundelfinger ED, Kreutz MR. 2013. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell. 152(5):1119-1133. https://doi.org/10.1016/j.cell.2013.02.002
Krautwald K, Min HK, Lee KH, Angenstein F. 2013. Synchronized electrical stimulation of the rat medial forebrain bundle and perforant pathway generates an additive BOLD response in the nucleus accumbens and prefrontal cortex. NeuroImage. 77:14-25. https://doi.org/10.1016/j.neuroimage.2013.03.046
Lützkendorf R, Hertel F, Heidemann R, Thiel A, Luchtmann M, Plaumann M, Stadler J, Baecke S, Bernarding J. 2013. Non-invasive high-resolution tracking of human neuronal pathways: Diffusion tensor imaging at 7T with 1.2 mm isotropic voxel size. in Progress in Biomedical Optics and Imaging. SPIE. https://doi.org/10.1117/12.2006764
Mallow J, Herrmann T, Kim KN, Stadler J, Mylius J, Brosch M, Bernarding J. 2013. Ultra-high field MRI for primate imaging using the travelling-wave concept. Magnetic Resonance Materials in Physics, Biology, and Medicine. 26(4):389-400. https://doi.org/10.1007/s10334-012-0358-z
Mylius J, Brosch M, Scheich H, Budinger E. 2013. Subcortical auditory structures in the mongolian gerbil: I. Golgi architecture. Journal of Comparative Neurology. 521(6):1289-1321. https://doi.org/10.1002/cne.23232
Puschmann S, Brechmann A, Thiel CM. 2013. Learning-dependent plasticity in human auditory cortex during appetitive operant conditioning. Human Brain Mapping. 34(11):2841-2851. https://doi.org/10.1002/hbm.22107
Riedel A, Stöber F, Richter K, Fischer KD, Miettinen R, Budinger E. 2013. VGLUT3-immunoreactive afferents of the lateral septum: Ultrastructural evidence for a modulatory role of glutamate. Brain Structure and Function. 218(1):295-301. https://doi.org/10.1007/s00429-012-0395-4
Sakaba T, Kononenko NL, Bacetic J, Pechstein A, Schmoranzer J, Yao L, Barth H, Shupliakov O, Kobler O, Aktories K, Haucke V. 2013. Fast neurotransmitter release regulated by the endocytic scaffold intersectin. Proceedings of the National Academy of Sciences of the United States of America. 110(20):8266-8271. https://doi.org/10.1073/pnas.1219234110
Schoonderwoert V, Dijkstra R, Luckinavicius G, Kobler O, van der Voort H. 2013. Huygens STED Deconvolution Increases Signal-to-Noise and Image Resolution towards 22 nm. Microscopy Today. 21(06):38 - 44. https://doi.org/10.1017/S1551929513001089
Selezneva E, Deike S, Knyazeva S, Scheich H, Brechmann A, Brosch M. 2013. Rhythm sensitivity in macaque monkeys. Frontiers in Systems Neuroscience. 7(SEP):Article 49. https://doi.org/10.3389/fnsys.2013.00049
Stirnweiss A, Hartig R, Gieseler S, Lindquist JA, Reichardt P, Philipsen L, Simeoni L, Poltorak M, Merten C, Zuschratter W, Prokazov Y, Paster W, Stockinger H, Harder T, Gunzer M, Schraven B. 2013. T cell activation results in conformational changes in the Src family kinase Lck to induce its activation. Science Signaling. 6(263):Article ra13. https://doi.org/10.1126/scisignal.2003607
Szycik GR, Stadler J, Brechmann A, Münte TF. 2013. Preattentive mechanisms of change detection in early auditory cortex: A 7Tesla fMRI study. Neuroscience. 253:100-109. https://doi.org/10.1016/j.neuroscience.2013.08.039
Tacikowski P, Brechmann A, Nowicka A. 2013. Cross-modal pattern of brain activations associated with the processing of self- and significant other's name. Human Brain Mapping. 34(9):2069-2077. https://doi.org/10.1002/hbm.22048
Traue HC, Ohl F, Brechmann A, Schwenker F, Kessler H, Limbrecht K, Hoffmann H, Scherer S, Kotzyba M, Scheck A, Walter S. 2013. A Framework for Emotions and Dispositions in Man-Companion Interaction. In Coverbal Synchrony in Human-Machine Interaction. CRC Press. pp. 99-140. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.986.1222&rep=rep1&type=pdf
Vielhaber S, Debska-Vielhaber G, Peeva V, Schoeler S, Kudin AP, Minin I, Schreiber S, Dengler R, Kollewe K, Zuschratter W, Kornblum C, Zsurka G, Kunz WS. 2013. Mitofusin 2 mutations affect mitochondrial function by mitochondrial DNA depletion. Acta Neuropathologica. 125(2):245-256. https://doi.org/10.1007/s00401-012-1036-y
Wanger T, Takagaki K, Lippert MT, Goldschmidt J, Ohl FW. 2013. Wave propagation of cortical population activity under urethane anesthesia is state dependent. Neuroscience. 14:Article 78. https://doi.org/10.1186/1471-2202-14-78
Weis T, Brechmann A, Puschmann S, Thiel CM. 2013. Feedback that confirms reward expectation triggers auditory cortex activity. Journal of Neurophysiology. 110(8):1860-1868. https://doi.org/10.1152/jn.00128.2013
Weis T, Puschmann S, Brechmann A, Thiel CM. 2013. Positive and negative reinforcement activate human auditory cortex. Frontiers in Human Neuroscience. 7(DEC):Article 842. https://doi.org/10.3389/fnhum.2013.00842
Yang S, Yang Z, Fischer K, Zhong K, Stadler J, Godenschweger F, Steiner J, Heinze HJ, Bernstein HG, Bogerts B, Mawrin C, Reutens DC, Speck O, Walter M. 2013. Integration of ultra-high field MRI and histology for connectome based research of brain disorders. Frontiers in Neuroanatomy. 7(SEP):Article 31. https://doi.org/10.3389/fnana.2013.00031
2012
Angenstein N, Scheich H, Brechmann A. 2012. Interaction between bottom-up and top-down effects during the processing of pitch intervals in sequences of spoken and sung syllables. NeuroImage. 61(3):715-722. https://doi.org/10.1016/j.neuroimage.2012.03.086
Apostolova I, Wunder A, Dirnagl U, Michel R, Stemmer N, Lukas M, Derlin T, Gregor-Mamoudou B, Goldschmidt J, Brenner W, Buchert R. 2012. Brain perfusion SPECT in the mouse: Normal pattern according to gender and age. NeuroImage. 63(4):1807-1817. https://doi.org/10.1016/j.neuroimage.2012.08.038
Bethmann A, Scheich H, Brechmann A. 2012. The Temporal Lobes Differentiate between the Voices of Famous and Unknown People: An Event-Related fMRI Study on Speaker Recognition. PLoS ONE. 7(10):e47626. https://doi.org/10.1371/journal.pone.0047626
Budinger E, Heil P. 2012. Anatomy of the auditory cortex. in Listening to Speech: An Auditory Perspective. Taylor & Francis Group. S. 91-113. https://doi.org/10.4324/9780203933107
Deike S, Heil P, Böckmann-Barthel M, Brechmann A. 2012. The build-up of auditory stream segregation: A different perspective. Frontiers in Psychology. 3(OCT):Article Article 461. https://doi.org/10.3389/fpsyg.2012.00461
Esparza J, Scherer S, Brechmann A, Schwenker F. 2012. Automatic emotion classification vs. human perception: Comparing machine performance to the human benchmark. in 2012 11th International Conference on Information Science, Signal Processing and their Applications, ISSPA 2012. S. 1253-1258. https://doi.org/10.1109/ISSPA.2012.6310484
Haroon F, Händel U, Angenstein F, Goldschmidt J, Kreutzmann P, Lison H, Fischer KD, Scheich H, Wetzel W, Schlüter D, Budinger E. 2012. Toxoplasma gondii actively inhibits neuronal function in chronically infected mice. PLoS ONE. 7(4):e35516. https://doi.org/10.1371/journal.pone.0035516
Hoffmann MB, Kaule FR, Levin N, Masuda Y, Kumar A, Gottlob I, Horiguchi H, Dougherty RF, Stadler J, Wolynski B, Speck O, Kanowski M, Liao YJ, Wandell BA, Dumoulin SO. 2012. Plasticity and Stability of the Visual System in Human Achiasma. Neuron. 75(3):393-401. https://doi.org/10.1016/j.neuron.2012.05.026
Kohrs C, Angenstein N, Scheich H, Brechmann A. 2012. Human striatum is differentially activated by delayed, omitted, and immediate registering feedback. Frontiers in Human Neuroscience. 6(AUGUST). https://doi.org/10.3389/fnhum.2012.00243
Krautwald K, Angenstein F. 2012. Low frequency stimulation of the perforant pathway generates anesthesia-specific variations in neural activity and BOLD responses in the rat dentate gyrus. Journal of Cerebral Blood Flow and Metabolism. 32(2):291-305. https://doi.org/10.1038/jcbfm.2011.126
Lovell J, Brosch M, Budinger E, Goldschmidt J, Scheich H, Tschorn S, Wendt B, Zuschratter W. 2012. Scanning and Transmission Electron Microscope Examination of Cochlea Hair and Pillar Cells from the Ear of the Mongolian Gerbil (Meriones unguiculatus). Anatomy and Physiology. 2(3).
Lutti A, Stadler J, Josephs O, Windischberger C, Speck O, Bernarding J, Hutton C, Weiskopf N. 2012. Robust and fast whole brain mapping of the RF transmit field B1 at 7T. PLoS ONE. 7(3):e32379. https://doi.org/10.1371/journal.pone.0032379
Macharadze T, Pielot R, Wanger T, Scheich H, Gundelfinger ED, Budinger E, Goldschmidt J, Kreutz MR. 2012. Altered neuronal activity patterns in the visual cortex of the adult rat after partial optic nerve crush-a single-cell resolution metabolic mapping study. Cerebral Cortex. 22(8):1824-1833. https://doi.org/10.1093/cercor/bhr256
Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, Tempelmann C, Speck O, Bogerts B, Abler B, Walter M. 2012. High field fMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. in BASAL GANGLIA CIRCUITS. Frontiers Media S. A. S. 197-213. https://doi.org/10.3389/978-2-88919-019-5
Schreiber S, Bueche CZ, Garz C, Kropf S, Angenstein F, Goldschmidt J, Neumann J, Heinze HJ, Goertler M, Reymann KG, Braun H. 2012. The pathologic cascade of cerebrovascular lesions in SHRSP: Is erythrocyte accumulation an early phase. Journal of Cerebral Blood Flow and Metabolism. 32(2):278-290. https://doi.org/10.1038/jcbfm.2011.122
Siegmund J, Brechmann A, Apel S, Kästner C, Liebig J, Leich T, Saake G. 2012. Toward measuring program comprehension with functional magnetic resonance imaging. in AMC SIGSOFT. https://doi.org/10.1145/2393596.2393624
Szycik GR, Stadler J, Tempelmann C, Münte TF. 2012. Examining the McGurk illusion using high-field 7 tesla functional MRI. Frontiers in Human Neuroscience. 6(APRIL 2012). https://doi.org/10.3389/fnhum.2012.00095
Tiede R, Krautwald K, Fincke A, Angenstein F. 2012. NMDA-dependent mechanisms only affect the BOLD response in the rat dentate gyrus by modifying local signal processing. Journal of Cerebral Blood Flow and Metabolism. 32(3):570-584. https://doi.org/10.1038/jcbfm.2011.182
Wanger T, Scheich H, Ohl FW, Goldschmidt J. 2012. The use of thallium diethyldithiocarbamate for mapping CNS potassium metabolism and neuronal activity: Tl +-redistribution, Tl +-kinetics and Tl +-equilibrium distribution. Journal of Neurochemistry. 122(1):106-114. https://doi.org/10.1111/j.1471-4159.2012.07757.x
Weber A, Prokazov Y, Zuschratter W, Hauser MJB. 2012. Desynchronisation of Glycolytic Oscillations in Yeast Cell Populations. PLoS ONE. 7(9):e43276. https://doi.org/10.1371/journal.pone.0043276
Weis T, Puschmann S, Brechmann A, Thiel CM. 2012. Effects of L-dopa during Auditory Instrumental Learning in Humans. PLoS ONE. 7(12):e52504. https://doi.org/10.1371/journal.pone.0052504
Wolff S, Brechmann A. 2012. MOTI: A motivational prosody corpus for speech-based tutorial systems. in Proceedings of 10th ITG Symposium on Speech Communication. Institute of Electrical and Electronics Engineers Inc.
You W, Achard S, Stadler J, Brückner B, U. S. 2012. Fractal analysis of resting state functional connectivity of the brain. in 2012 International Joint Conference on Neural Networks, IJCNN 2012. Article 6252657. https://doi.org/10.1109/IJCNN.2012.6252657
2011
Brechmann A, Brosch M, Budinger E, Heil P, König R, Ohl F, Scheich H. 2011. Auditory cortex - Current concepts in human and animal research. Hearing Research. 271(1-2):1-2. https://doi.org/10.1016/j.heares.2010.10.016
Börzsönyi T, Unger T, Szabó B, Wegner S, Angenstein F, Stannarius R. 2011. Reflection and exclusion of shear zones in inhomogeneous granular materials. Soft Matter. 7(18):8330-8336. https://doi.org/10.1039/c1sm05762f
Coomber B, Edwards D, Jones SJ, Shackleton TM, Goldschmidt J, Wallace MN, Palmer AR. 2011. Cortical inactivation by cooling in small animals. Frontiers in Systems Neuroscience. 5(JUNE 2011):53. https://doi.org/10.3389/fnsys.2011.00053
Engel K, Toennies KD, Brechmann A. 2011. Part-based localisation and segmentation of landmark-related auditory cortical regions. Pattern Recognition. 44(9):2017-2033. https://doi.org/10.1016/j.patcog.2010.09.004
Heidt T, Deininger F, Peter K, Goldschmidt J, Pethe A, Hagemeyer CE, Neudorfer I, Zirlik A, Weber WA, Bode C, Meyer PT, Behe M, von zur Mühlen C. 2011. Activated platelets in carotid artery thrombosis in mice can be selectively targeted with a radiolabeled single-chain antibody. PLoS ONE. 6(3):e18446. https://doi.org/10.1371/journal.pone.0018446
Hutton C, Josephs O, Stadler J, Featherstone E, Reid A, Speck O, Bernarding J, Weiskopf N. 2011. The impact of physiological noise correction on fMRI at 7T. NeuroImage. 57(1):101-112. https://doi.org/10.1016/j.neuroimage.2011.04.018
Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, Medvedev N, Wilczek E, De Roo M, Zuschratter W, Muller D, Wilczynski GM, Mozrzymas JW, Stewart MG, Kaczmarek L, Wlodarczyk J. 2011. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. Journal of Cell Science. 124(19):3369-3380. https://doi.org/10.1242/jcs.090852
Nowicka A, Marchewka A, Jednoróg K, Tacikowski P, Brechmann A. 2011. Forgetting of emotional information is hard: An fMRI study of directed forgetting. Cerebral Cortex. 21(3):539-549. https://doi.org/10.1093/cercor/bhq117
Scheich H, Brechmann A, Brosch M, Budinger E, Ohl FW, Selezneva E, Stark H, Tischmeyer W, Wetzel W. 2011. Behavioral semantics of learning and crossmodal processing in auditory cortex: The semantic processor concept. Hearing Research. 271(1-2):3-15. https://doi.org/10.1016/j.heares.2010.10.006
Tacikowski P, Brechmann A, Marchewka A, Jednoróg K, Dobrowolny M, Nowicka A. 2011. Is it about the self or the significance? an fMRI study of self-name recognition. Social Neuroscience. 6(1):98-107. https://doi.org/10.1080/17470919.2010.490665
Tyll S, Budinger E, Noesselt T. 2011. Thalamic influences on multisensory integration. Communicative and Integrative Biology. 4(4):378-381. https://doi.org/10.4161/cib.15222
Vitali M, Picazo F, Prokazov Y, Duci A, Turbin E, Götze C, Llopis J, Hartig R, Visser AJWG, Zuschratter W. 2011. Wide-Field multi-parameter FLIM: Long-term minimal invasive observation of proteins in living cells. PLoS ONE. 6(2):e15820. https://doi.org/10.1371/journal.pone.0015820
2010
Angenstein F, Krautwald K, Scheich H. 2010. The current functional state of local neuronal circuits controls the magnitude of a BOLD response to incoming stimuli. NeuroImage. 50(4):1364-1375. https://doi.org/10.1016/j.neuroimage.2010.01.070
Bachmann A, Kobler O, Kittel RJ, Wichmann C, Sierralta J, Sigrist SJ, Gundelfinger ED, Knust E, Thomas U. 2010. A perisynaptic ménage à trois between Dlg, DLin-7, and metro controls proper organization of Drosophila synaptic junctions. Journal of Neuroscience. 30(17):5811-5824. https://doi.org/10.1523/JNEUROSCI.0778-10.2010
Deike S, Scheich H, Brechmann A. 2010. Active stream segregation specifically involves the left human auditory cortex. Hearing Research. 265(1-2):30-37. https://doi.org/10.1016/j.heares.2010.03.005
Goldschmidt J, Wanger T, Engelhorn A, Friedrich H, Happel M, Ilango A, Engelmann M, Stuermer IW, Ohl FW, Scheich H. 2010. High-resolution mapping of neuronal activity using the lipophilic thallium chelate complex TlDDC: Protocol and validation of the method. NeuroImage. 49(1):303-315. https://doi.org/10.1016/j.neuroimage.2009.08.012
Hamhaber U, Klatt D, Papazoglou S, Hollmann M, Stadler J, Sack I, Bernarding J, Braun J. 2010. In vivo magnetic resonance elastography of human brain at 7 T and 1.5 T. Journal of Magnetic Resonance Imaging. 32(3):577-583. https://doi.org/10.1002/jmri.22294
Hammer N, Steinke H, Böhme J, Stadler J, Josten C, Spanel-Borowski K. 2010. Description of the iliolumbar ligament for computer-assisted reconstruction. Annals of Anatomy. 192(3):162-167. https://doi.org/10.1016/j.aanat.2010.02.003
Hammer R, Brechmann A, Ohl F, Weinshall D, Hochstein S. 2010. Differential category learning processes: The neural basis of comparison-based learning and induction. NeuroImage. 52(2):699-709. https://doi.org/10.1016/j.neuroimage.2010.03.080
Herrmann CS, Brechmann A, Scheich H. 2010. Simultaneous EEG and fMRI of the human auditory system. in EEG - fMRI: Physiological Basis, Technique, and Applications. Springer. S. 385-399. https://doi.org/10.1007/978-3-540-87919-0_19
Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, Tempelmann C, Speck O, Bogerts B, Abler B, Walter M. 2010. High field fMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Frontiers in Neuroanatomy. 4(NOV). https://doi.org/10.3389/fnana.2010.00138
Noesselt T, Tyll S, Boehler CN, Budinger E, Heinze HJ, Driver J. 2010. Sound-induced enhancement of low-intensity vision: Multisensory influences on human sensory-specific cortices and thalamic bodies relate to perceptual enhancement of visual detection sensitivity. Journal of Neuroscience. 30(41):13609-13623. https://doi.org/10.1523/JNEUROSCI.4524-09.2010
Steinke H, Hammer N, Slowik V, Stadler J, Josten C, Böhme J, Spanel-Borowski K. 2010. Novel insights into the sacroiliac joint ligaments. Spine. 35(3):257-263. https://doi.org/10.1097/BRS.0b013e3181b7c675
Thomas U, Kobler O, Gundelfinger ED. 2010. The Drosophila larval neuromuscular junction as a model for scaffold complexes at glutamatergic synapses: Benefits and limitations. Journal of Neurogenetics. 24(3):109-119. https://doi.org/10.3109/01677063.2010.493589
Wittenbrink N, Weber TS, Klein A, Weiser AA, Zuschratter W, Sibila M, Schuchhardt J, Or-Guil M. 2010. Broad volume distributions indicate nonsynchronized growth and suggest sudden collapses of germinal center B cell populations. Journal of Immunology. 184(3):1339-1347. https://doi.org/10.4049/jimmunol.0901040
- Third Party Funds
Third Party Funds
2023-2028
NFDI4BIOIMAGE - National Research Data Infrastructure for Microscopy and Bioimage Analysis. Task Area 3: Multimodal data linking and integration. (Stöter, Zuschratter)
nfdi4bioimage.de2022-2024
Leibniz Cooperative Excellence: "Learning Resilience: Supporting neuronal network state transitions to foster stress resilience" WP 3 Brain-wide network state transitions associated with cognitive flexibility in the framework of stress resilience. (Budinger, Goldschmidt)
2021-2025
DFG CRC 1436 "Neural Resources of Cognition", Project B06 “Mobilisation of neural resources for temporal attention” (Budinger, Noesselt, Pakan)
www.iknd.ovgu.de/SFB+1436.html2021-2024
DFG “Lateralization and hemispheric interaction during auditory processing in children with ADHD” (Angenstein)
BMBF "Joint project: Time Resolved Raman and Metabolic Imaging Spectroscopy Unit (TIRAMISU) - Sub project: Exploration and evaluation of a high-resolution hybrid camera system for Fluorescence Lifetime Imaging in combination with Fluorescence and Raman Spectroscopy" (Zuschratter)2020-2022
CBBS NeuroNetwork “Non-invasive Deep Brain Stimulation for Motor Disorders (NeeMo)” (Budinger, Ruhnau, Carius)
2019-2021
DFG “Linking program comprehension to neural, behavioral, and psycho-physiological correlates” (Brechmann, Siegmund)
2018-2021
DFG “Multimodal recognition of affect over the course of a tutorial learning experiment” (Brechmann, Schwenker)
2010-2021
DFG CRC 854 “Molecular organisation of cellular communication within the immune system”, TP Z01 “Multimodal Imaging Platform” (Zuschratter, Dudeck, Müller)
www.sfb854.de2018-2020
EU EFRE “Intentional Antizipatory Interaktive Systems” TP2 “Neural mechnisms of information processing in dialogs” (Brechmann)
2017-2020
EU Horizon 2020: Corbel shared services for life-science: “Functional 3D analysis of immune synapse contacts”, PID 2376 (Zuschratter)
2016-2020
BMWi Exist Transfer of Research: Photonscore – ultra-sensitive and ultra-fast research camera, FKZ Exist 03EFGST025 (Prokazov, Turbin, Zuschratter)
BMWi Exist funding phase I: 2016 -2017: BMWi Exist funding phase II:2018-2020
2016-2020
EU ESF “ABINEP - Analysis, Imaging, and Modelling of Neuronal and Inflammatory Processes”, Module 1: Neuroinflammation: Inflammatory processes in neurodegeneration; Project 2: Development of new techniques for visualization of neuroinflammatory processes during infections and autoimmunity diseases of the brain (Budinger, Goldschmidt, Gopola, Schlüter)
www.abinep.ovgu.de2016-2019
DFG “Understanding Program Comprehension in the Neuro-Imaging Age” (Brechmann, Siegmund)
DFG CRC 779, Project B13 “Emotional aspects of event learning in rats: Characterization and neural basis” (Fendt, Goldschmidt)
www.sfb779.de2012-2019
DFG “Combinatorial NeuroImaging Core Facility” (Brechmann, Zuschratter)
2011-2019
DFG “Hemispheric interaction during lateralized auditory processing in humans: effects of task difficulty, training and age” (Angenstein)
2016-2018
European Regional Development Fund (ERDF) and State of Saxony Anhalt “Settlement of a 9.4 T small animal magnetic resonance tomograph”
europa.sachsen-anhalt.de/esi-fonds-in-sachsen-anhalt/esi-fonds-in-sachsen-anhalt2015-2018
BMBF-Network “EmoAdapt” TP “Disposition-adaptive user support based on neural activity and psychophysiology” (Brechmann)
2009-2017
DFG CRC-TRR 62 “Companion Technology”, TP B2 “Neural mechanisms of information processing in dialogs” (Brechmann, Scheich)
2005-2017
DFG CRC-TRR 31 “The Active Auditory System”, TP A4 “Predictive mechanisms in active stream segregation and related tasks” (Brechmann, Brosch, Scheich)
2013-2016
BMBF Consortium project: Minimal-invasive, multi-parameter wide-field microscopy, Sub-project: Exploration and Evaluating of multi-anode camera system, FKZ: 13N12675 (Zuschratter)
BMBF VIP project “201TlDDC-SPECT for early diagnosis of dementia diseases” (Goldschmidt, Reymann)
2013-2014
BMBF Research campus STIMULATE “Detection of Photons”, FKZ 03FO16101C (Zuschratter, Prokazov, Turbin)
2012-2013
EFRE “Procurement of a 3 Tesla magnetic resonance imaging system”
2009-2013
BMBF “Magdeburger Institut für Demenzforschung (MID)”, Project “Category training in elderly normal and cognitively impaired subjects” (Scheich, Brechmann)
DFG CRC-TRR 31 “The Active Auditory System”, TP A13 “Functional anatomy of interhemispheric and thalamic interactions of the auditory cortex” (Budinger)
2009-2012
BMBF Consortium project: Quantum, Sub-project: Exploration and evaluation of an ultra-sensitive, time-resolving camera system, FKZ: 13N10077 (Zuschratter)
DFG CRC-TRR 31 “The Active Auditory System”, TP A12 “Dopaminergic modulation of learning-dependent plasticity in the audiotry cortex” (Thiel, Brechmann)
2009-2010
DAAD exchange programme: “Identification of vesicular glutamate transporter 3-positive synapses in the lateral septum of the rat” (Miettien, Riedel, Budinger)
2008-2010
LSA, EU “Neurobiologically inspired, multimodal intention recognition for technical systems”, Project “Mechanisms of prosody processing in dialogs” (Scheich, Brechmann)
2007-2010
BMBF-Network “How does the brain distinguish between prosody and song”, Project “Auditory processing of prosody and song” (Scheich, Brechmann)
- Transfer
LINCam: Development of an ultra-sensitive, time-resolving research camera for functional imaging (Yury Prokazov, Evgeny Turbin, André Weber, Werner Zuschratter)
In addition to spatial and temporal resolution, the sensitivity of detection poses the greatest challenge in the microscopic examination of living cells and tissues. Taken into account that all existing microscope systems put living samples enormously under stress, we have developed a minimally invasive imaging system for the visualization of biosensors in native cells and tissues. The technical realization resulted in a patented and award-winning ultra-sensitive, time-resolved wide-field quantum detector with a wide range of applications. It has been used successfully in several research projects for FLIM and FRET measurements of biosensors (Stirnweiss et al. 2013; Philipson et al. 2017), but also for determination of pH and Ca2+ sensors, label-free metabolic imaging (Weber et al., 2017), single molecule detection (Oleksiievets et al., 2020), quantum optics and Raman spectroscopy (Konugolu et al., 2018). Since the camera system displayed a major market potential, the spin-off “Photonscore GmbH” was founded in 2017 that successfully markets the technology since then under the name “LINCam”.
Figure: camera modules of LINCam25 and LINCam40Figure: LINCam25 mounted on a Leica Lightsheet microscope
Funding:- BMBF TCAM4Life (FKZ: 13N12675)
- BMWi EXIST (FKZ: 03EFGST025)
- Teaching
Teaching
Teaching & Internships
We supervise internships as well as bachelor, master, and doctoral theses. If you are interested, please contact us!
We regularly give lectures and seminars for students of the Master Program "Integrative Neuroscience", the Master Program Psychology, the Bachelor Program Philosophy, Neurowissenschaften, Kognition, and the Bachelor Program Computer Science
In addition, we conduct workshops on image analysis and, in cooperation with the Neuroscientific Society (NWG), hold annual courses on the functional organization of synapses.
Specific courses:- MicroImaging, Lecture and Practical Course in the Master Program Integrative Neuroscience
- Macroimaging, Lecture in the Master Program Integrative Neuroscience
- Advanced Statistics for Neuroscience, Lecture and Exercise in the, Master Program Integrative Neuroscience
- Neuroanatomy, Lecture in the Master Program Psychology
- Introduction to Nervous Systems, Lecture in the Bachelor Program Philosophy, Neurowissenschaften, Kognition
- Neurobiological Learning Research, Seminar and Lab Rotation in the Bachelor Program Computer Science, Learning Systems / Biocomputing
- Summerschool of the Bachelor Program Computer Science, Learning Systems / Biocomputing