Department Cellular Neuroscience
Research topics
- cracking the neuronal code
cracking the neuronal code
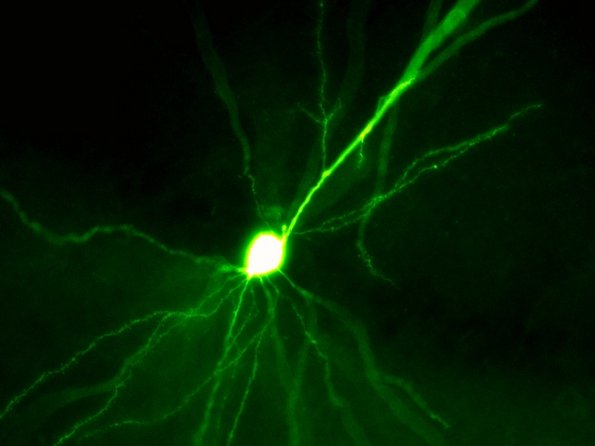
We want to understand how hippocampal neurons in the subiculum generate the neuronal code of action potential sequences that is transmitted to other brain regions such as the cortex. This action potential code is densely packed with information on the spatial position, self-motion, motivation, salience and is tuned by prior experience via synaptic plasticity. For cracking this hippocampal output code, we monitor synaptic input of multiple kinds during the behavior. We then use computational modeling to predict the conversion of input to output and finally test our predictions by recording directly from single neurons with synaptic resolution during behavior (supported by ERC consolidator grant SUB-D-Code). – Dennis Dalügge, Oliver Barnstedt, Pavol Bauer, Hiroshi Kaneko
- using artificial intelligence to detect features in neural activity and behavior
using artificial intelligence to detect features in neural activity and behavior
We are using machine vision and learning to classify behavior. The application of artificial intelligence based tools has the potential to revolutionize research on neural correlates of behavior. In particular, we classify the way an animal changes its pose and interacts with an environment in an unsupervised manner. We are convinced that high temporal resolution unsupervised behavioral classification will be a first step allowing us to correlate behavioral features with neural activity patterns. The second step is extracting scientific understanding and detecting causal relationships within these large behavior-correlated neural activity imaging data sets. In other words, we want to understand biological neural networks by using artificial neural networks as a tool. (supported by EFRE funding, Pavol Bauer, Kevin Luxem, Johannes Kürsch)
- understanding the diversity of neurons
understanding the diversity of neurons
For most brain areas a comprehensive understanding of the cell clusters forming the neural circuits is currently absent. In collaboration with Nelson Spruston (HHMI Janelia), Mark Cembrowski (University of Vancouver and the Janelia Quantitative Genomics Unit we use single-cell RNA sequencing and multiplexed in situ hybridization for transcriptomic profiling of cell types. A brain region of highest interest is the medial septum. It is a brain region that controls hippocampal memory circuits. We have set up an innovative framework of bioinformatics analyses to identify genetically-defined cell populations. In combination with viral tracing tools we further reveal specific hippocampus-projecting clusters and dissect their function during behavior (supported by HHMI Janelia Visitor Project) – Stefano Pupe
- understanding vulnerability and resilience of neural circuits to cognitive dysfunction
understanding vulnerability and resilience of neural circuits to cognitive dysfunction
We are investigating how hippocampal memory circuits and individual neurons within these circuits respond to progressive pathological challenges. Here we have set a focus on the accumulation of amyloid beta, which can be observed in human Alzheimer patients and in advanced physiological aging. We found a computational principle explaining why reduced dendritic size in the response to pathology results in higher neuronal. By using computational modeling, we could understand how neuronal structural changes define electrical properties of dendrites and entire neurons. On the circuit level, we are investigating how hippocampal inhibitory and excitatory neurons interact in microcircuits and how altered interneuron function contributes to the malfunction of learning and memory processes (supported by SFB 1089 project B01) – Liudmila Sosulina, Hiroshi Kaneko
- investigating the subcortical modulation of memory circuits
investigating the subcortical modulation of memory circuits
Subcortical areas form the core of our brain, they are involved in complex activities such as memory, emotion, pleasure, arousal and hormone production. They act as hubs by relaying and modulating information passing to different areas of the brain including the hippocampus. We are trying to gain a better understanding on how hippocampal circuits are controlled by direct and indirect subcortical modulation from the medial septum, the locus coeruleus and the ventral tegmental area. The medial septum is a basal forebrain region that is reciprocally connected with the hippocampal formation, where it orchestrates microcircuit activation and oscillatory activity during different behavioral states. It is known as a main source of Acetylcholine, which is released by septo-hippocampal afferents during learning and memory processes. We have discovered that, in addition to cholinergic projections, glutamatergic and GABAergic septo-hippocampal projections play a key role in the control of activity levels of hippocampal neurons during memory guided navigation. The medial septum is an important network hub, it integrates behavioral state dependent input from locus coeruleus and channels specific information to the hippocampus and other memory-relevant brain regions. We want to better understand the multitude of medial septal functions during behavior (supported by SFB 1089 subproject C05) – Petra Mocellin, Liudmila Sosulina
The department
- News
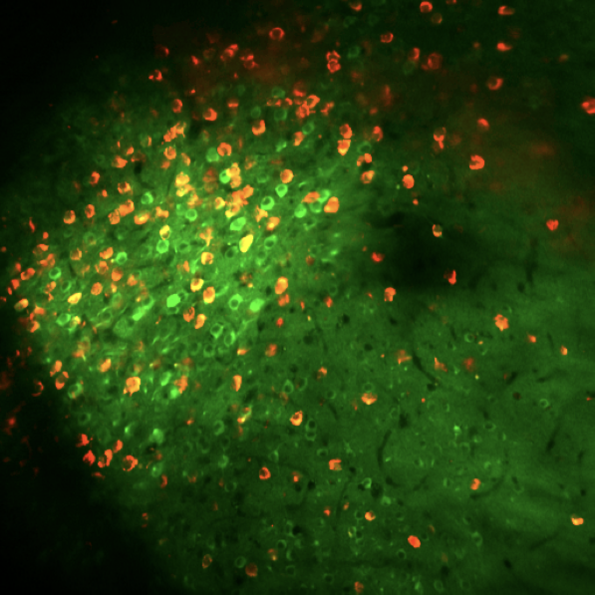
Preprint "Hippocampal hyperactivity in a rat model of Alzheimer’s disease". This illustration shows amyloid deposits (in green) in the vicinity of a CA1 pyramidal neuron. The deposition of beta amyloid is a hallmark of human Alzheimer’s disease. In our work (Sosulina et al. bioRxiv 2020, Siskova et al. Neuron 2014) we discovered new pathomechanisms of how neurons change their excitability in the presence of amyloid beta. On the network level these changes result in aberrant patterns of oscillations ( in the local field potential, in red). Preprint entitled “Identifying Behavioral Structure from Deep Variational Embeddings of Animal Motion” News
New preprint on mechanisms of neuronal hyperexcitability and network dysfunction in Alzheimer’s disease
Our new paper is out. It ist the result of a great collaboration with Martin Fuhrmann’s (DZNE ) and Michael Rowan’s (Trinity College, Dublin) labs. Liudmila Sosulina and Manuel Mittag analyzed the hippocampal micronetwork in a rat model of AD at an early disease stage at the beginning of extracellular amyloid beta (Aβ) deposition. They are the first to show with two-photon Ca2+-imaging in vivo and patch-clamp physiology that neuronal hyperexcitability can be observed in the hippocampus of rats and is present at very early stages. They show that mechanistically this micronetwork dysfunction is due to changes of intrinsic neuronal excitability, rather than reduced inhibitory synaptic transmission, which sets in at later stages. Our data support the view that altered intrinsic excitability of CA1 neurons may precede inhibitory dysfunction at an early stage of disease progression.
Release of new preprint including research code
We are excited to share a new preprint entitled “Identifying Behavioral Structure from Deep Variational Embeddings of Animal Motion” that is available at BioRxiv.
In this work we present a novel machine learning framework for discovery of the latent structure of animal behavior given data from markerless pose estimation entitled Variational Animal Motion Embedding (VAME). VAME clusters the time series data into “behavioral motifs”, which are stereotypical behavioral patterns that occur repeatedly in free behavior. The structure of behavior is then represented in the distribution of motifs and the probability of transitions between them. In the preprint we show that this information is sensitive enough to serve as a basis for classification of mice according to its behavior phenotypes. This can be done even for subtle behavioral differences, which human experts are unable to detect from the video capture.
- Head

Head
Since January 2020 Stefan Remy is the scientific director of the LIN and the head of the Department of Cellular Neuroscience. He is also appointed as Professor of Molecular and Cellular Neurobiology at the Otto von Guericke University, Medical Faculty in Magdeburg.
Stefan Remy’s department investigates how neural computations on the cellular and circuit level underlie memory guided behavior and cognitive dysfunction. Within the department biologists, mathematicians and computer scientists collaborate to extract scientific understanding from multidimensional behavioral and neurophysiological data sets.
He obtained the doctoral degree at the University of Bonn in 2003 and then worked from 2003-2005 as postdoc with Heinz Beck at the Department of Epileptology (Head: Christian E. Elger). As an Alexander-von-Humboldt fellow he joined the Department of Neurobiology and Physiology at Northwestern University, Evanston, in 2005. There he worked with Nelson Spruston on dendritic excitability and synaptic plasticity. In 2007 he continued his postdoctoral training with Heinz Beck in Bonn, where he started his own research group in 2009 funded by the state of NRW. From 2010 he headed a research group at the German Center of Neurodegenerative Diseases Bonn (Head: Pierluigi Nicotera) before moving to Magdeburg in 2020.
There are places I remember
All my life though some have changed
Some forever not for better
Some have gone and some remain
All these places have their moments
With lovers and friends I still can recall
Some are dead and some are living
In my life I've loved them all.(John Lennon/ Paul McCartney)
My research philosophy is explained here in my inaugural lecture.
- Members
Members
Head Prof. Dr. Stefan Remy +49 391 6263 92421, 92411, 93311 stefan.remy@lin-magdeburg.de Assistent Juliane Jäger +49 391 6263 92411 juliane.jaeger@lin-magdeburg.de Research group leaders
Dr. Alessio Attardo (Hippocampal plasticity and memory Group)
+49 391 6263 93371 alessio.attardo@lin-magdeburg.de Prof. Dr. Stefan Remy (Cells and Circuits)
+49 391 6263 92421, 92411, 93311 stefan.remy@lin-magdeburg.de Postdocs Dr. Oliver Barnstedt (DZNE Bonn)
Dr. Stefan Dürschmid (Sensory learning and prediction)
+49 391 6263 92431 stefan.duerschmid@lin-magdeburg.de Dr. Hiroshi Kaneko
+49 391 6263 93371 hiroshi.kaneko@lin-magdeburg.de Dr. Liudmila Sosulina
+49 391 6263 93371 liudmila.sosulina@lin-magdeburg.de Dr. Ulrich Thomas (Functional Genetics of the Synapse)
+49 391 6263 93231 ulrich.thomas@lin-magdeburg.de PhD students Petra Mocellin
+49 391 6263 9 2411 petra.mocellin@lin-magdeburg.de Dennis Dalügge +49 391 6263 93361 dennis.daluegge@lin-magdeburg.de Damaris Holder
+49 391 6263 93321 damaris.holder@lin-magdeburg.de Felix Kuhn
+49 391 6263 93321 felix.kuhn@lin-magdeburg.de Kevin Luxem
+49 391 6263 93361 kevin.luxem@lin-magdeburg.de Lab coordinators/Research Technicians
Jorge R. Bergado Acosta +49 391 6263 93271 jorge.bergado-acosta@lin-magdeburg.de Janina Juhle +49 391 6263 93321 janina.juhle@lin-magdeburg.de Silvia Vieweg +49 391 6263 93481 silvia.vieweg@lin-magdeburg.de Students and other guests Dr. Falko Fuhrmann (Lab consultant)
- Publications
Publications
Key publications
Luxem K, Fuhrmann F, Kürsch J, Remy S*, Bauer P*. 2020. Identifying Behavioral Structure from Deep Variational Embeddings of Animal Motion. BioRxiv. https://doi.org/10.1101/2020.05.14.095430 * shared senior authorship
Justus D, Dalügge D, Bothe S, Fuhrmann F, Hannes C, Kaneko H, Friedrichs D, Sosulina L, Schwarz I, Elliott DA, Schoch S, Bradke F, Schwarz MK, Remy S. 2017. Glutamatergic synaptic integration of locomotion speed via septoentorhinal projections. Nature Neuroscience. 20(1):16-19. https://doi.org/10.1038/nn.4447
Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis HR, Schwarz I, Schmidt B, Schwarz MK, Remy S, Fuhrmann M. 2016. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer's Disease Model. Neuron. 92(1):114-125. https://doi.org/10.1016/j.neuron.2016.08.034
Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. 2015. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron. 86(5):1253-1264. https://doi.org/10.1016/j.neuron.2015.05.001
Šišková Z, Justus D, Kaneko H, Friedrichs D, Henneberg N, Beutel T, Pitsch J, Schoch S, Becker A, vonderKammer H, Remy S. 2014. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of alzheimer's disease. Neuron. 84(5):1023-1033. https://doi.org/10.1016/j.neuron.2014.10.024
Müller C, Beck H, Coulter D, Remy S. 2012. Inhibitory Control of Linear and Supralinear Dendritic Excitation in CA1 Pyramidal Neurons. Neuron. 75(5):851-864. https://doi.org/10.1016/j.neuron.2012.06.025
Krueppel R, Remy S, Beck H. 2011. Dendritic integration in hippocampal dentate granule cells. Neuron. 71(3):512-528. https://doi.org/10.1016/j.neuron.2011.05.043
Park JY, Remy S, Varela J, Cooper DC, Chung S, Kang HW, Lee JH, Spruston N. 2010. A post-burst afterdepolarization is mediated by group I metabotropic glutamate receptor-dependent upregulation of Cav2.3 R-type calcium channels in CA1 pyramidal neurons. PLoS Biology. 8(11):e1000534. https://doi.org/10.1371/journal.pbio.1000534
Remy S, Csicsvari J, Beck H. 2009. Activity-Dependent Control of Neuronal Output by Local and Global Dendritic Spike Attenuation. Neuron. 61(6):906-916. https://doi.org/10.1016/j.neuron.2009.01.032
Remy S, Spruston N. 2007. Dendritic spikes induce single-burst long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 104(43):17192-17197. https://doi.org/10.1073/pnas.0707919104
All publications of the Department
2023
Luxem K, Sun JJ, Bradley SP, Krishnan K, Yttri E, Zimmermann J, Pereira TD, Laubach M, Cai DJ, Colgin LL. 2023. Open-source tools for behavioral video analysis: Setup, methods, and best practices. eLife. 12:e79305. https://doi.org/10.7554/eLife.79305
Barnstedt O, Mocellin P, Remy S. 2023. A hippocampus-accumbens code guides goal-directed appetitive behavior. bioRxiv. https://doi.org/10.1101/2023.03.09.531869
Beckmann D, Langnaese K, Gottfried A, Hradsky J, Tedford K, Tiwari N, Thomas U, Fischer K-D, Korthals M. 2023. Ca2+ Homeostasis by Plasma Membrane Ca2+ ATPase (PMCA) 1 Is Essential for the Development of DP Thymocytes. International Journal of Molecular Sciences. 24(2):Article 1442. https://doi.org/10.3390/ijms24021442
Ghelani T, Escher M, Thomas U, Esch K, Lützkendorf J, Depner H, Maglione M, Parutto P, Gratz S, Matkovic-Rachid T, Ryglewski S, Walter AM, Holcman D, O'Connor Giles K, Heine M, Sigrist SJ 2023. Interactive nanocluster compaction of the ELKS scaffold and Cacophony Ca2+ channels drives sustained active zone potentiation. Science advances. 9(7):Article eade7804. https://doi.org/10.1126/sciadv.ade7804
Grochowska KM, Gomes GM, Raman R, Kaushik R, Sosulina L, Kaneko H, Oelschlegel AM, Yuanxiang P, Reyes-Resina I, Bayraktar G, Samer S, Spilker C, Woo MS, Morawski M, Goldschmidt J, Friese MA, Rossner S, Navarro G, Remy S, ... Kreutz MR. 2023. Jacob-induced transcriptional inactivation of CREB promotes Aβ-induced synapse loss in Alzheimer's disease. The EMBO journal. 42(4):Article e112453. https://doi.org/10.15252/embj.2022112453
Holder D, Prigge M. 2023. Spatial and Temporal Considerations of Optogenetic Tools in an All-Optical Single-Beam Experiment. Papagiakoumou E, editor. In All-Optical Methods to Study Neuronal Function. Humana Press Inc. pp. 165-185. (Neuromethods). https://doi.org/10.1007/978-1-0716-2764-8_6
Mittag M, Mediavilla L, Remy S, Cuntz H, Jedlicka P. 2023. Modelling the contributions to hyperexcitability in a mouse model of Alzheimer's disease. Journal of Physiology. https://doi.org/10.1113/JP283401
2022
Chenani A, Weston G, Ulivi AF, Castello-Waldow TP, Huettl R-E, Chen A, Attardo A. 2022. Repeated stress exposure leads to structural synaptic instability prior to disorganization of hippocampal coding and impairments in learning. Translational Psychiatry. 12(1):Article 381. https://doi.org/10.1038/s41398-022-02107-5
Eckert D, Reichert C, Bien CG, Heinze H-J, Knight RT, Deouell LY, Dürschmid S. 2022. Distinct interacting cortical networks for stimulus-response and repetition-suppression. Communications biology. 5(1):Article 909. https://doi.org/10.1038/s42003-022-03861-4
Krueger J, Krauth R, Reichert C, Perdikis S, Vogt S, Huchtemann T, Dürschmid S, Sickert A, Lamprecht J, Huremovic A, Goertler M, Nasuto SJ, Knight RT, Hinrichs H, Heinze H-J, Lindquist S, Sailer M, Millán JDR, Sweeney-Reed CM. 2022. Functional electrical stimulation driven by a brain–computer interface in acute and subacute stroke patients impacts beta power and long-range temporal correlation. In 2022 IEEE Workshop on Complexity in Engineering (COMPENG). IEEE. https://doi.org/10.1109/COMPENG50184.2022.9905448
Luxem K, Mocellin P, Fuhrmann F, Kürsch J, Miller SR, Palop JJ, Remy S, Bauer P. 2022. Identifying behavioral structure from deep variational embeddings of animal motion. Communications biology. 5(1):Article 1267. https://doi.org/10.1038/s42003-022-04080-7
Luxem K, Mocellin P. 2022. Self-supervised learning as a gateway to reveal underlying dynamics in animal behavior. Sprink A, Barski J, Brouwer A-M, Riedel G, Sil A, editors. In Volume 2 of the Proceedings of the joint 12th International Conference on Methods and Techniques in Behavioral Research and 6th Seminar on Behavioral Methods held online May 18-22 2022. pp. 167-170. https://doi.org/10.6084/m9.figshare.20066849.v2
Luxem K, Sun JJ, Bradley SP, Krishnan K, Yttri EA, Zimmermann J, Pereira TD, Laubach M. 2022. Open-Source Tools for Behavioral Video Analysis: Setup, Methods, and Development. arXiv. https://doi.org/10.48550/arXiv.2204.02842
Reichert C, Dürschmid S, Sweeney-Reed CM, Hinrichs H. 2022. Visual spatial attention shifts decoded from the electroencephalogram enable sending of binary messages. In 2022 IEEE Workshop on Complexity in Engineering, COMPENG 2022. IEEE. https://doi.org/10.1109/COMPENG50184.2022.9905445
2021
Kobler O, Weiglein A, Hartung K, Chen Y-C, Gerber B, Thomas U. 2021. A quick and versatile protocol for the 3D visualization of transgene expression across the whole body of larval Drosophila. Journal of Neurogenetics. 35(3):306-319. https://doi.org/10.1080/01677063.2021.1892096
Korthals M, Tech L, Langnaese K, Gottfried A, Hradsky J, Thomas U, Zenclussen AC, Brunner-Weinzierl MC, Tedford K, Fischer KD. 2021. Plasma membrane Ca2+ ATPase 1 (PMCA1) but not PMCA4 is critical for B-cell development and Ca2+ homeostasis in mice. European Journal of Immunology. 51(3):594-602. https://doi.org/10.1002/eji.202048654
Korvasová K, Ludwig F, Kaneko H, Sosulina L, Tetzlaff T, Remy S, Mikulovic S. 2021. Locomotion induced by medial septal glutamatergic neurons is linked to intrinsically generated persistent firing. bioRxiv. https://doi.org/10.1101/2021.04.23.441122
Krick N, Ryglewski S, Pichler A, Bikbaev A, Götz T, Kobler O, Heine M, Thomas U, Duch C. 2021. Separation of presynaptic Cav2 and Cav1 channel function in synaptic vesicle exo- and endocytosis by the membrane anchored Ca2+ pump PMCA. Proceedings of the National Academy of Sciences of the United States of America. 118(28):Article e2106621118. https://doi.org/10.1073/pnas.2106621118
Mocellin P, Mikulovic S. 2021. The Role of the Medial Septum-Associated Networks in Controlling Locomotion and Motivation to Move. Frontiers in neural circuits. 15:Article 699798. https://doi.org/10.3389/fncir.2021.699798
Sarkar I, Maji I, Omprakash C, Stober S, Mikulovic S, Bauer P. 2021. Evaluation of deep lift pose models for 3D rodent pose estimation based on geometrically triangulated data. CV4Animals Workshop, CVPR 2021. https://arxiv.org/abs/2106.12993
Sosulina L, Mittag M, Geis H-R, Hoffmann K, Klyubin I, Qi Y, Steffen J, Friedrichs D, Henneberg N, Fuhrmann F, Justus D, Keppler K, Cuello AC, Rowan MJ, Fuhrmann M, Remy S. 2021. Hippocampal hyperactivity in a rat model of Alzheimer's disease. Journal of Neurochemistry. 157(6):2128-2144. https://doi.org/10.1111/jnc.15323
Wachtler T, Bauer P, Denker M, Grün S, Hanke M, Klein J, Oeltze-Jafra S, Ritter P, Rotter S, Scherberger H, Stein A, Witte OW. 2021. NFDI-Neuro: Building a community for neuroscience research data management in Germany. Neuroforum. 27(1):3-15. https://doi.org/10.1515/nf-2020-0036
2020
Bertan F, Wischhof L, Sosulina L, Mittag M, Dalügge D, Fornarelli A, Gardoni F, Marcello E, Di Luca M, Fuhrmann M, Remy S, Bano D, Nicotera P. 2020. Loss of Ryanodine Receptor 2 impairs neuronal activity-dependent remodeling of dendritic spines and triggers compensatory neuronal hyperexcitability. Cell Death and Differentiation. 27(12):3354-3373. https://doi.org/10.1038/s41418-020-0584-2
Luxem K, Fuhrmann F, Kürsch J, Remy S*, Bauer P*. 2020. Identifying Behavioral Structure from Deep Variational Embeddings of Animal Motion. bioRxiv. https://doi.org/10.1101/2020.05.14.095430 * shared senior authorship
2019Schwarz MK, Remy S. 2019. Rabies virus-mediated connectivity tracing from single neurons. Journal of Neuroscience Methods. 325:108365. https://doi.org/10.1016/j.jneumeth.2019.108365
Luxem K, Fuhrmann F, Remy S,Bauer P. 2019. Hierarchical network analysis of behavior and neuronal population activity. In: 2019 Conference on Cognitive Computational Neuroscience. Berlin, Germany: Cognitive Computational Neuroscience; 2019. https://doi.org/10.32470/CCN.2019.1261-0
2018Dalügge D, Remy S. 2018. Human Cortical Dendrites: Stretched to Perform Better?. Cell. 175(3):635-637. https://doi.org/10.1016/j.cell.2018.09.052
Giovannetti E, Poll S, Justus D, Kaneko H, Fuhrmann F, Steffen J, Remy S, Fuhrmann M. 2018. Restoring memory by optogenetic synchronization of hippocampal oscillations in an Alzheimer’s disease mouse model. BioRXiv (preprint). https://doi.org/10.1101/363820
Musial TF, Molina-Campos E, Bean LA, Ybarra N, Borenstein R, Russo ML, Buss EW, Justus D, Neuman KM, Ayala GD, Mullen SA, Voskobiynyk Y, Tulisiak CT, Fels JA, Corbett NJ, Carballo G, Kennedy CD, Popovic J, Ramos-Franco J, Fill M, Pergande MR, Borgia JA, Corbett GT, Pahan K, Han Y, Chetkovich DM, Vassar RJ, Byrne RW, Matthew Oh M, Stoub TR, Remy S, Disterhoft JF, Nicholson DA. 2018. Store depletion-induced h-channel plasticity rescues a channelopathy linked to Alzheimer's disease. Neurobiology of Learning and Memory. 154:141-157. https://doi.org/10.1016/j.nlm.2018.06.004
Müller C, Geis HR, Remy S. 2018. Visually Guided Single-Cell Recordings in the Hippocampus of Awake Mice. Manahan-Vaughan D, editor. In Handbook of in Vivo Neural Plasticity Techniques. Elsevier B.V. pp. 123-134. (Handbook of Behavioral Neuroscience). https://doi.org/10.1016/B978-0-12-812028-6.00006-9
Müller C, Remy S. 2018. Septo–hippocampal interaction. Cell and Tissue Research. 373(3):565-575. https://doi.org/10.1007/s00441-017-2745-2
2017Justus D, Dalügge D, Bothe S, Fuhrmann F, Hannes C, Kaneko H, Friedrichs D, Sosulina L, Schwarz I, Elliott DA, Schoch S, Bradke F, Schwarz MK, Remy S. 2017. Glutamatergic synaptic integration of locomotion speed via septoentorhinal projections. Nature Neuroscience. 20(1):16-19. https://doi.org/10.1038/nn.4447
Remy S, Poirazi P, Papoutsi A. 2017. Introduction to the Computational Neuroscience Special Section. European Journal of Neuroscience. 45(8):998-999. https://doi.org/10.1111/ejn.13562
2016Müller C, Remy S. 2016. Slowly Building Excitement. Cell. 165(7):1568-1569. https://doi.org/10.1016/j.cell.2016.06.005
Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis HR, Schwarz I, Schmidt B, Schwarz MK, Remy S, Fuhrmann M. 2016. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer's Disease Model. Neuron. 92(1):114-125. https://doi.org/10.1016/j.neuron.2016.08.034
2015Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. 2015. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron. 86(5):1253-1264. https://doi.org/10.1016/j.neuron.2015.05.001
Wagner J, Krauss S, Shi S, Ryazanov S, Steffen J, Miklitz C, Leonov A, Kleinknecht A, Göricke B, Weishaupt JH, Weckbecker D, Reiner AM, Zinth W, Levin J, Ehninger D, Remy S, Kretzschmar HA, Griesinger C, Giese A, Fuhrmann M. 2015. Reducing tau aggregates with anle138b delays disease progression in a mouse model of tauopathies. Acta Neuropathologica. 130(5):619-631. https://doi.org/10.1007/s00401-015-1483-3
2014Müller C, Remy S. 2014. Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Frontiers in Synaptic Neuroscience. 6(SEP):23. https://doi.org/10.3389/fnsyn.2014.00023
Pothmann L, Müller C, Averkin RG, Bellistri E, Miklitz C, Uebachs M, Remy S, de la Prida LM, Beck H. 2014. Function of inhibitory micronetworks is spared by Na+ channel-acting anticonvulsant drugs. Journal of Neuroscience. 34(29):9720-9735. https://doi.org/10.1523/JNEUROSCI.2395-13.2014
Qi Y, Klyubin I, Harney SC, Hu NW, Cullen WK, Grant MK, Steffen J, Wilson EN, Do Carmo S, Remy S, Fuhrmann M, Ashe KH, Cuello AC, Rowan MJ. 2014. Longitudinal testing of hippocampal plasticity reveals the onset and maintenance of endogenous human Aß-induced synaptic dysfunction in individual freely behaving pre-plaque transgenic rats: Rapid reversal by anti-Aß agents. Acta neuropathologica communications. 2(1):175. https://doi.org/10.1186/s40478-014-0175-x
Šišková Z, Justus D, Kaneko H, Friedrichs D, Henneberg N, Beutel T, Pitsch J, Schoch S, Becker A, vonderKammer H, Remy S. 2014. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of alzheimer's disease. Neuron. 84(5):1023-1033. https://doi.org/10.1016/j.neuron.2014.10.024
2013Müller C, Remy S. 2013. Fast micro-iontophoresis of glutamate and GABA: a useful tool to investigate synaptic integration. Journal of visualized experiments: JoVE. (77). https://doi.org/10.3791/50701
2012Müller C, Beck H, Coulter D, Remy S. 2012. Inhibitory Control of Linear and Supralinear Dendritic Excitation in CA1 Pyramidal Neurons. Neuron. 75(5):851-864. https://doi.org/10.1016/j.neuron.2012.06.025
2011Krueppel R, Remy S, Beck H. 2011. Dendritic integration in hippocampal dentate granule cells. Neuron. 71(3):512-528. https://doi.org/10.1016/j.neuron.2011.05.043
Chen S, Su H, Yue C, Remy S, Royeck M, Sochivko D, Opitz T, Beck H, Yaari Y. 2011. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. Journal of Neurophysiology. 105(1):117-129. https://doi.org/10.1152/jn.00184.2010
2010Park JY, Remy S, Varela J, Cooper DC, Chung S, Kang HW, Lee JH, Spruston N. 2010. A post-burst afterdepolarization is mediated by group I metabotropic glutamate receptor-dependent upregulation of Cav2.3 R-type calcium channels in CA1 pyramidal neurons. PLoS Biology. 8(11):e1000534. https://doi.org/10.1371/journal.pbio.1000534
Remy S, Beck H, Yaari Y. 2010. Plasticity of voltage-gated ion channels in pyramidal cell dendrites. Current Opinion in Neurobiology. 20(4):503-509. https://doi.org/10.1016/j.conb.2010.06.006
2009Remy S, Csicsvari J, Beck H. 2009. Activity-Dependent Control of Neuronal Output by Local and Global Dendritic Spike Attenuation. Neuron. 61(6):906-916. https://doi.org/10.1016/j.neuron.2009.01.032
2008Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. 2008. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. Journal of Neurophysiology. 100(4):2361-2380. https://doi.org/10.1152/jn.90332.2008
2007Remy S, Spruston N. 2007. Dendritic spikes induce single-burst long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 104(43):17192-17197. https://doi.org/10.1073/pnas.0707919104
2006Remy S, Beck H. 2006. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 129(Pt 1):18-35. https://doi.org/10.1093/brain/awh682
Heinemann U, Kann O, Remy S, Beck H. 2006. Novel mechanisms underlying drug resistance in temporal lobe epilepsy. Advances in neurology. 97:85-95.
2005Yue C, Remy S, Su H, Beck H, Yaari Y. 2005. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. Journal of Neuroscience. 25(42):9704-9720. https://doi.org/10.1523/JNEUROSCI.1621-05.2005
2004Remy C, Remy S, Beck H, Swandulla D, Hans M. 2004. Modulation of voltage-dependent sodium channels by the δ-agonist SNC80 in acutely isolated rat hippocampal neurons. Neuropharmacology. 47(7):1102-1112. https://doi.org/10.1016/j.neuropharm.2004.06.034
2003Ellerkmann RK, Remy S, Chen J, Sochivko D, Elger CE, Urban BW, Becker A, Beck H. 2003. Molecular and functional changes in voltage-dependent Na+ channels following pilocarpine-induced status epilepticus in rat dentate granule cells. Neuroscience. 119(2):323-333. https://doi.org/10.1016/S0306-4522(03)00168-4
Remy S, Urban BW, Elger CE, Beck H. 2003. Anticonvulsant pharmacology of voltage-gated Na+ channels in hippocampal neurons of control and chronically epileptic rats. European Journal of Neuroscience. 17(12):2648-2658. https://doi.org/10.1046/j.1460-9568.2003.02710.x
Remy S, Gabriel S, Urban BW, Dietrich D, Lehmann TN, Elger CE, Heinemann U, Beck H. 2003. A novel mechanism underlying drug resistance in chronic epilepsy. Annals of Neurology. 53(4):469-479. https://doi.org/10.1002/ana.10473
- Methods
Methods
Our machine-learning based mouse behavioral classification paradigm (VAME) can detect behavioral motifs from spatiotemporal video data. The video camera monitors the mouse through the glass bottom of an open field arena. 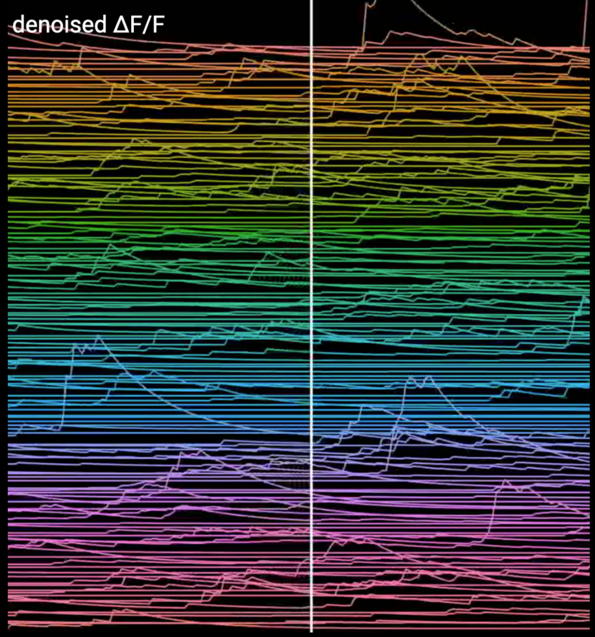
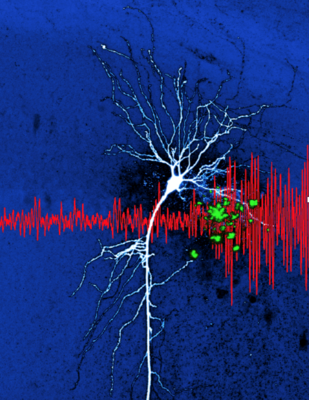
These traces represent changes of the intracellular calcium concentration of a neuronal population in a hippocampal subregion. In these two-photon imaging experiments the change of fluorescence upon calcium binding by the GCamP6 molecules serves as a proxy of neuronal activity (as calcium is entering the cell with every action potential). 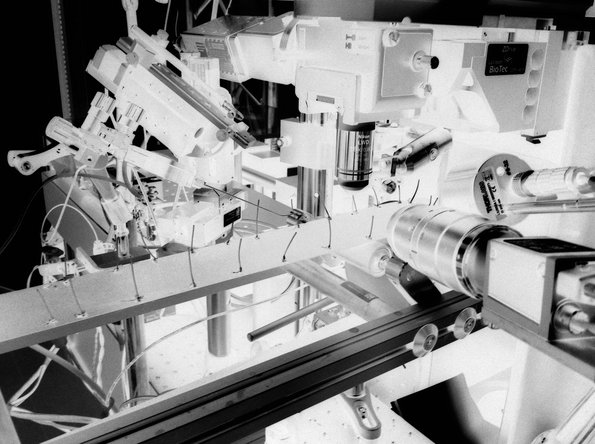
The image shows a combined behavioral and two-photon microscopy setup that is used in our laboratory. 
©Fuhrmann et al. NEURON, In a virtual reality paradigm mice can perform spatial learning tasks by navigating the environment displayed by the screens. - Third Party Funds



Third Party Funds
Projects of the department are supported by:
- Open Science
Open Science
public data of the department
- The data set includes detailed electrophysiological characterizations of cell types in the medial entorhinal cord (layer 2/3).
- This GitHub repository contains the research code of the VAME framework as well as documentation and exemplary data: Variational Animal Motion Embedding (VAME)

![[Translate to English:]](/fileadmin/_processed_/a/8/csm_Bild6_3922802f9e.png)