Hippocampal plasticity and memory Group
The ability to store and consciously recall past experiences is crucial for survival and establishing a coherent sense of reality. This ability in mammals is dependent on the hippocampal formation, which encodes experience through coordinated neuronal activity. We want to dissect the cellular and circuits mechanisms underlying hippocampus' ability to acquire and recall memories. We use intravital optical imaging to study neuronal synaptic plasticity and the neuronal activity patterns underlying experience representation in the hippocampus of live mice performing learning tasks. We take advantage of molecular and genetic tools to investigate defined synapses and manipulate activity of specific neuronal populations. Together these tools enable us to investigate the cellular events in the hippocampus underlying learning and recall going from molecular mechanisms to network-level computation.
Please find more information here.
- Head
Head

Alessio Attardo is a neurobiologist fascinated by how neurons store and compute information. He is interested in how networks of neurons in the brain acquire and recall memories.
After completing his undergraduate studies in biology at the University of Palermo (Italy), in 2002 he moved to the Max Planck Institute of Cell Biology and Genetics in Dresden (Germany) to start his Ph.D. training under the supervision of Wieland B. Huttner. During that time he described the lineage of progenitors that constitute the major source of neurons in the mammalian neocortex. In 2008 he moved to California (U.S.A.) as a postdoctoral fellow. At Stanford University, under the supervision of Mark J. Schnitzer, he developed a novel chronic deep-brain optical imaging technique to study long-term plasticity of pyramidal neurons in the hippocampus of live mice. In 2015, he started his own independent research group within the Department of Stress Neurobiology and Neurogenetics at the Max Planck Institute for Psychiatry in Munich (Germany), focusing on structural plasticity in hippocampal neurons as the cellular foundation of memory and on the effects of stress on structural plasticity and neuronal representations.
Since January 2022, Alessio Attardo is leading his group - Hippocampal plasticity and episodic memory -within the Department of Cellular Neuroscience at the LIN.
- Members
Members
Head Dr. Alessio Attardo +49-391-6263-93371 alessio.attardo@lin-magdeburg.de Postdocs Dr. Leonid Fedorov +49 391 6263 93371 leonid.fedorov@lin-magdeburg.de Dr. Hadi Mirzapourdelavar +49-391-6263-93351 hadi.mirzapourdelavar@lin-magdeburg.de PhD students Hannah Klimmt +49-391-6263-93351 hannah.klimmt@lin-magdeburg.de Bhargavi Keerthana Boovaraga Murthy +49-391-6263-93351 bhargavi.murthy@lin-magdeburg.de Stefanos Somatakis +49 391 6263 92411 stefanos.somatakis@lin-magdeburg.de Alumni Lasse Büchen Akilashree Senthilnathan Alessandro Ulivi - Research interests
Research interests
Synptic plasticity and memory
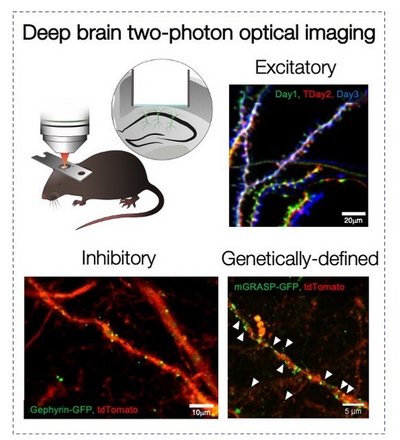
It is widely assumed that synapses are a basic substrate of memory. While in neocortex, two-photon imaging is casting light on the relationship between learning and synaptic turnover, such a relationship is still obscure in the hippocampus. We want to establish a direct link between synaptic plasticity and learning in the hippocampal CA1 of live animals, and to examine how stress influences this function. To this aim we use optical imaging of dendritic spines in the basal aspect of hippocampal CA1 (Attardo et al., 2015; Ulivi et al., 2019 and Castello-Waldow et al., 2020) to investigate long-term synaptic turnover in live animals learning to perform a memory task that requires the hippocampus.
Neuronal representations and memory
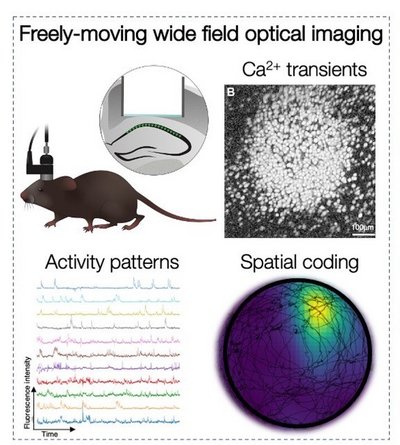
Excitatory and inhibitory neurons represent spatial and non-spatial information by their activity patterns in hippocampal CA1. How do these patterns emerge? What is their function during learning and recall? How does stress change them? To answer these and more questions we use microscopes that can be permanently mounted on the mouse head to record activity of hundreds of CA1 neurons as mice perform hippocampal-dependent memory tasks.
- Third party funds
Third party funds
2024-2027
DFG research grant
AT205/18-1: “Stability of synaptic memory engram“
2021-2024
DFG research grant
AT205/10-1: “How does activity of CA3 engram neurons affect CA1 spatial codes?“
2021-2024
DFG research grant
AT205/9-1: “Maintaining Activity Set-Points in the Hippocampus: From Long-Term Dynamics of Excitatory and Inhibitory Synapses to Functional Stability of CA1 Circuits“
2020-2023
DFG research grant
AT205/7-1: “The impact of neuronal activity on functional re wiring of hippocampal CA1 excitatory neurons”
2017-2020
Schram foundation research grant
T287/29575/2017: “Determining the function of local inhibitory circuits in the synaptic dynamics of hippocampal pyramidal neurons during learning and memory”
2017-2020
DFG research grant
AT205/1-1: “Investigating the effects of chronic stress on hippocampal representations, synaptic connectivity and learning” - Publications
Publications
Learning to become addicted, one synapse at a time. Attardo A, Cambridge SB. Neural Regen Res. 2024 Feb;19(2):401-402. 10.4103/1673-5374.379046
Arc-driven mGRASP highlights CA1 to CA3 synaptic engrams. Murthy BKB, Somatakis S, Ulivi AF, Klimmt H, Castello-Waldow TP, Haynes N, Huettl RE, Chen A, Attardo A. Front Behav Neurosci. 2023 Jan 30;16:1072571. 10.3389/fnbeh.2022.1072571. eCollection 2022
Repeated stress exposure leads to structural synaptic instability prior to disorganization of hippocampal coding and impairments in learning. Chenani A, Weston G, Ulivi AF, Castello-Waldow TP, Huettl RE, Chen A, Attardo A. Transl Psychiatry. 2022 Sep 12;12(1):381. 10.1038/s41398-022-02107-5.
Hippocampal neurons with stable excitatory connectivity become part of neuronal representations. Castello-Waldow TP, Weston G, Ulivi AF, Chenani A, Loewenstein Y, Chen A, Attardo A. PLoS Biol. 2020 Nov 3;18(11):e3000928. https://doi.org/10.1371/journal.pbio.3000928
Longitudinal Two-Photon Imaging of Dorsal Hippocampal CA1 in Live Mice. Ulivi AF, Castello-Waldow TP, Weston G, Yan L, Yasuda R, Chen A, Attardo A. J Vis Exp. 2019 Jun 19;(148). 10.3791/59598
Long-Term Consolidation of Ensemble Neural Plasticity Patterns in Hippocampal Area CA1. Attardo A, Lu J, Kawashima T, Okuno H, Fitzgerald JE, Bito H, Schnitzer MJ. Cell Rep. 2018 Oct 16;25(3):640-650.e2. https://doi.org/10.1016/j.celrep.2018.09.064
Impermanence of dendritic spines in live adult CA1 hippocampus. Attardo A, Fitzgerald JE, Schnitzer MJ. Nature. 2015 Jul 30;523(7562):592-6. 10.1038/nature14467. Epub 2015 Jun 22.
Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Barretto RP, Ko TH, Jung JC, Wang TJ, Capps G, Waters AC, Ziv Y, Attardo A, Recht L, Schnitzer MJ. Nat Med. 2011 Feb;17(2):223-8. https://doi.org/10.1038/nm.2292. Epub 2011 Jan 16.
Tis21 expression marks not only populations of neurogenic precursor cells but also new postmitotic neurons in adult hippocampal neurogenesis. Attardo A, Fabel K, Krebs J, Haubensak W, Huttner WB, Kempermann G. Cereb Cortex. 2010 Feb;20(2):304-14. https://doi.org/10.1093/cercor/bhp100. Epub 2009 May 29.
Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. Cereb Cortex. 2009 Oct;19(10):2439-50. https://doi.org/10.1093/cercor/bhn260. Epub 2009 Jan 23.
Cytokinesis of neuroepithelial cells can divide their basal process before anaphase. Kosodo Y, Toida K, Dubreuil V, Alexandre P, Schenk J, Kiyokage E, Attardo A, Mora-Bermúdez F, Arii T, Clarke JD, Huttner WB. EMBO J. 2008 Dec 3;27(23):3151-63. https://doi.org/10.1038/emboj.2008.227. Epub 2008 Oct 30.
Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. Attardo A, Calegari F, Haubensak W, Wilsch-Bräuninger M, Huttner WB. PLoS One. 2008 Jun 11;3(6):e2388. https://doi.org/10.1371/journal.pone.0002388.
Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Bonilla S, Hall AC, Pinto L, Attardo A, Götz M, Huttner WB, Arenas E. Glia. 2008 Jun;56(8):809-20. https://doi.org/10.1002/glia.20654
The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Bräuninger M, Eilken M, Schroeder T, Huttner W, Brakebusch C, Götz M. Nat Neurosci. 2006 Sep;9(9):1099-107. Epub 2006 Aug 6. 10.1038/nn1744
Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Haubensak W, Attardo A, Denk W, Huttner WB. Proc Natl Acad Sci USA. 2004 Mar 2;101(9):3196-201. Epub 2004 Feb 12. https://doi.org/10.1073/pnas.0308600100