Cognitive Neurophysiology
The hippocampus and entorhinal cortex, which are part of the medial temporal lobe (MTL), support memory and spatial navigation functions. Our research focuses on neural computation underlying cognitive functions of the MTL, combining in vitro and in vivo electrophysiological recordings with computational simulations. Since the MTL is crucially involved in Alzheimer’s disease and temporal lobe epilepsy, studying neural computation in the MTL is central not only to understanding of cognitive functions and its engineering applications, but also to developing treatments for dementia and epilepsy.
- Head
Head

Motoharu Yoshida studied Information Technology at the Kyushu Institute of Technology in Fukuoka (Japan) and holds a PhD title from there, too. He then did postdoctoral research at McGill University (Montreal Neurological Institute) and at the Center for Memory and Brain (Boston University).
Subsequently, Yoshida worked as a junior professor at the Ruhr University in Bochum. He has been with LIN and DZNE Magdeburg since 2016.
- Members
Members
Head Dr. Motoharu Yoshida +49-391-6263-94011 motoharu.yoshida@lin-magdeburg.de Technical staff member and lab service Dr. Antonio Reboreda Prieto +49-391-6263-94501 antonio.prieto@lin-magdeburg.de PhD students Yacine Brahimi Simone Calabrese Alan Tobias Price Alumni Babak Saber Marouf - Projects
Projects
The group investigates electrophysiological properties of neurons as a fundamental element of cognitive function. Temporal association memory
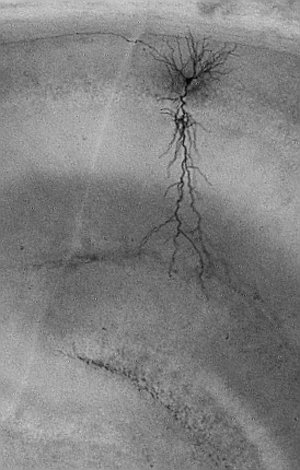
The ability to associate events that are temporally apart, requires an intact hippocampus. Our current major focus is on the roles of transient receptor potential cation (TRPC) channels in temporal association memory. TRPC channels are abundant in hippocampal principal cells (Fig. 1A). We have recently shown that TRPC channels modulate dynamical properties of hippocampal pyramidal cells known as persistent firing (Fig. 1B). Computational simulations have indicated that TRPC channels play crucial roles in shaping neural network activity, allowing persistent firing to co-exist with oscillatory dynamics. We plan to further our understandings of the contributions of TRPC channels on behavioral levels using TRPC channel manipulations in vivo.
Spatial navigation
Grid cells in the medial entorhinal cortex (MEC) are believed to support spatial navigation. It has been proposed that grid cell firing emerges through path integration, which is the ability to update spatial representation by using idiothetic cues. MEC layer II has mainly two different types of cells: pyramidal and stellate cells. However, the cellular mechanisms supporting path integration and grid cell formation remain unknown. Oscillatory properties of MEC layer II neurons and persistent firing may support path integration (Fig. 2). We have shown that properties of MEC layer II neurons such as the spike adaptation ratio are different along the dorso-ventral axis, indicating these cellular properties may underlie differences in grid cell spacing. We further continue this line of research to identify cellular mechanism of path integration from cellular to behavioral levels.
Memory encoding and consolidation
It has been suggested that the MTL supports memory formation through two distinct processes: encoding and consolidation. Hippocampal neurons switch their firing mode from "place cell" or "time cell" activity during encoding to the temporally compressed "replay" activity during consolidation. The prevailing theory suggests that the level of the neuromodulator acetylcholine triggers this transition of operational modes. Our theoretical and computational work suggests that cholinergic modulations of intrinsic cellular properties, including the TRPC and potassium channels, may support the transition.More information are available here: yoshida-lab.weebly.com/our-research.html
- Selected Publications
Selected Publications
Valero-Aracama MJ, Reboreda A, Arboit A, Sauvage M, Yoshida M (2021) Noradrenergic Suppression of Persistent Firing in Hippocampal CA1 Pyramidal Cells through cAMP-PKA Pathway. eNeuro, 8(2). doi: https://pubmed.ncbi.nlm.nih.gov/33637539/
Arboit A, Reboreda A, Yoshida M (2020) Involvement of TRPC4 and 5 channels in persistent firing in hippocampal CA1 pyramidal cells, Cells, 9(2). doi: https://pubmed.ncbi.nlm.nih.gov/32033274/
Knauer B, Yoshida M (2019) Switching between persistent firing and depolarization block in individual rat CA1 pyramidal neurons. Hippocampus, 29(9):817-835. doi: https://onlinelibrary.wiley.com/doi/full/10.1002/hipo.23078
Reboreda A, Theissen F, Arboit A, Corbu MA, Valero-Aracama M, Yoshida M (2018) Do TRPC channels support working memory?: Comparing modulations of TRPC channels and working memory through G-protein coupled receptors and neuromodulators, Behav Brain Res., 354:64-83. doi: https://pubmed.ncbi.nlm.nih.gov/29501506/
Cutsuridis V and Yoshida M (2017) Editorial: Memory Processes in Medial Temporal Lobe: Experimental, Theoretical and Computational Approaches. Front. Syst. Neurosci. 11:19. doi: https://www.frontiersin.org/articles/10.3389/fnsys.2017.00019/full
Jochems A, Yoshida M. A robust in vivo-like persistent firing supported by the CAN current in a recurrent neural network. PLoS ONE. 2015 Jan 01; 10:e0123799. doi: 10.1371/journal.pone.0123799
Saravanan V, Arabali D, Jochems A, Cui AX, Gootjes-Dreesbach L, …, Yoshida M. Transition between encoding and consolidation/replay dynamics via cholinergic modulation of CAN current: a modelling study. Hippocampus. 2015 Jan 01; 25 doi: 10.1002/hipo.22429
Knauer B, Jochems A, Valero-Aracama MJ, Yoshida M. Long-lasting intrinsic persistent firing in rat CA1 pyramidal cells: a possible mechanism for active maintenance of memory. Hippocampus. 2013 Jan 01; 23:820-31. doi: 10.1002/hipo.22136
Yoshida M, Giocomo LM, Boardman I, Hasselmo ME. Frequency of subthreshold oscillations at different membrane potential voltages in neurons at different anatomical positions on the dorso-ventral axis in the rat medial entorhinal cortex. J Neurosci. 2011 Jan 01; 31:12683-94. doi: 10.1523/JNEUROSCI.1654-11.2011
Yoshida M, Hasselmo M. Persistent firing supported by an intrinsic cellular mechanism in a component of the head direction system. J Neurosci. 2009 Jan 01; 29:4945-4952. doi: 10.1523/JNEUROSCI.5154-08.2009
- Current Third Party Funds
Current Third Party Funds
2022- 2025
DFG grant
"Cellular mechanisms underlying short-term memory in the hippocampal CA1 region"